Microbial production of one of the organic feed stocks from plant substances such as molasses is presently used for ethanol production. This alcohol was produced by fermentation in the early days but for many years by chemical means through the catalytic hydration of ethylene.
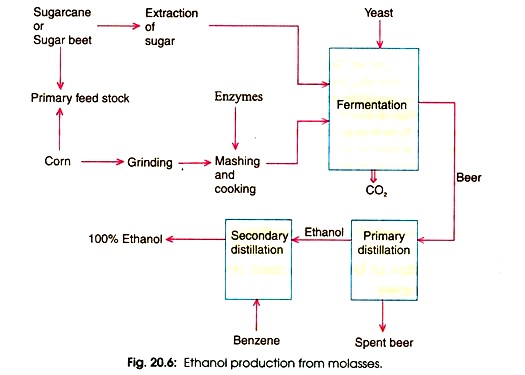
In modem era, attention has been paid to the production of ethanol for chemical and fuel purposes by microbial fermentation. Ethanol is now-a-days produced by using sugar beet, potatoes, com, cassava, and sugar cane (Fig. 20.6).
Both yeasts (Saccharomyces cerevisiae, S. uvarum S. carlsbergensis, Candida brassicae, C. utilis, Kluyveromyces fragilis, K. lactis) and bacteria (Zymomonas mobilis) have been employed for ethanol production in industries.
The commercial production is carried out with Saccharomyces cerevisiae. On the other hand, uvarum has also largely been used. The Candida utilis is used for the fermentation of waste sulphite liquor since it also ferments pentoses.
Recently, experimentation with Schizosaccharomyces has shown promising results. When whey from milk is used, strain of K. fragilis is recommended for the production of ethanol. It is also found that Fusarium, Bacillus and Pachysolen tannophilus (yeast) can transform pentose sugars to ethanol.
Theoretically, it is interesting to note that fermentation process retains most of the energy of the sugar in the form of ethanol. The heat of combustion of solid sucrose is 5.647 MJ mol-1, the heat of combustion of glucose is 2.816 MJ mol-1 but the heat release is 1.371 MJ mol-1.
The equations are given below:
Thus the above reactions show that 97% sugar transforms into ethanol. But in practice, the fermentation yield of ethanol from sugar is about 46% or one hundred grams of pure glucose will yield 48.4 grams of ethanol, 46.6 g of CO2, 3.3 grams of glycerol and 1.2 g of yeast. The biosynthesis of ethanol is given in Fig. 20.6.
It is noteworthy that the ethanol at high concentration inhibits the yeast. Hence, the concentration of ethanol reduces the yeast growth rate which affect the biosynthesis of ethanol.
It can produce about 10-12 % ethanol but the demerit of yeast is that it has limitation of converting whole biomass derived by their ability to convert xylulose into ethanol. The Zymomonas has a merit over yeast that it has osmotic tolerance to higher sugar concentration. It is relatively having high tolerance to ethanol and have more specific growth rate.
1. Preparation of Medium:
Three types of substrates are used for ethanol production:
(a) Starch containing substrate,
(b) Juice from sugarcane or molasses or sugar beet,
(c) Waste products from wood or processed wood. Production of ethanol from whey is not viable.
If yeast strains are to be used, the starch must be hydrolysed as yeast does not contain amylases. After hydrolysis, it is supplemented with celluloses of microbial origin so as to obtain reducing sugars. About 1 ton of starch required 1 litre of amylases and 3.5 litre of glucoamylases. Following steps are involved in conversion of starch into ethanol (Fig. 20.7).
On the other hand, if molasses are used for ethanol production, the bagasse can also give ethanol after fermentation. Several other non-conventional sources of energy such as aquatic plant biomass, wood after hydrolysis with celluloses gives ethanol. Sulphite waste-liquor, a waste left after production of paper, also contains hexose as well as pentose sugar. The former can be microbially easily converted.
2. Fermentation:
Ethanol is produced by continuous fermentation. Hence, large fermenters are used for continuous manufacturing of ethanol. The process varies from one country to another. India, Brazil, Germany, Denmark have their own technology for ethanol production.
The fermentation conditions are almost similar (pH 5, temperature 35°C) but the cultures and culture conditions are different. The fermentation is normally carried out for several days but within 12h starts production. After the fermentation is over, the cells are separated to get biomass of yeast cells which are used as single cell protein (SCP) for animal’s feed.
The culture medium or supernatant is processed for recovery of ethanol (Fig. 20.6). Ethanol is also produced by batch fermentation as no significant difference is found both in batch and continuous fermentation.
Although as stated earlier within 12h Saccharomyces cerevisiae starts producing ethanol at the rate of 10% (v/v) with 10-20g cells dry weight/lit. The reduction in fermentation time is accomplished use of ceil recycling continuously in fermentation.
3. Recovery:
Ethanol can be recovered upto 95 percent by successive distillations. To obtain 100 percent, it requires to form an azeotropic mixture containing 5 percent water. Thus 5 percent water is removed from azeotropic mixture of ethanol, water and benzene after distillation. In this procedure, benzene water ethanol and then ethanol-benzene azeotropic mixture are removed so that absolute alcohol is obtained.
Neuberg’s Fermentation:
Yeasts utilize pyruvate during fermentation resulting in the formation of an intermediary product acetaldehyde.
This is trapped by hydrogen sulfite to yield the acetaldehyde in precipitated form and fluid product formation is glycerol as shown below:
CH3 CHO + NaHSO3 → CH2-CHOH-SO3Na
Now in place of acetaldehyde, dihydroxyacetone phosphate acts as a hydrogen acceptor which is reduced to glycerol-3-phosphate.
After removal of phosphate i.e. dephosphorylation, it gives glycerol as given below:
C6H12O6 + H2SO3 → CH2-CHOH-SO3Na + Glycerol + CO2
Neuberg’s fermentation process is categorized as reward and third fermentation.
The first fermentation equation is given below:
2Glucose + H2O → C2H5OH + acetate + glycerol + 2CO2