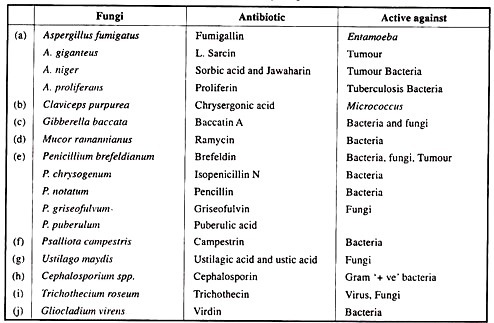
Inter-molecular forces of non-covalent bond between antigen and antibody is divided into four basic types:
Type # 1. Electrostatic Interaction:
The force between oppositely charged ionic groups on the two protein side chains is known as electrostatic forces (Fig. 5.2a). The force of attraction (F) is inversely proportional to the square of the distance (d) between the charges. For e.g., ionized amino groups (NH3+) on lysine of one protein and as ionized carboxyl group (—COO–) of glutamate on the other.
Attraction (F) α 1/Kd2 [K, dielectric constant]
Therefore, the closer the charges would increase attractive forces. This force can be generated by charge transfer reaction in between the antigen and antibody.
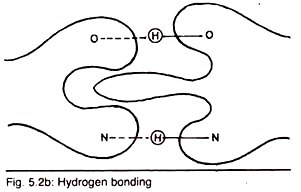
Type # 2. Hydrogen Bonding:
Another bonding type is hydrogen bond which is reversible bond between hydrophilic groups e.g., NH2, OH and COOH depend on the proximity of two molecules having these groups (Fig. 5.2b). It is a weak bond which involves the release of water between the reacting side chains.
Type # 3. Vander-Waals Forces:
Vander-Waals force is greatly influenced by presence of external electron cloud. Ideal gases like hydrogen and nitrogen exhibit this bond between them. The force of attraction (F) is inversely related to the seventh power of distance (d) between two molecules (Fig. 5.2c).
Force (F) α 1/d7
Type # 4. Hydrophobic Interaction:
Hydrophobic, non-polar group’s; like leucine, valine and phenyl-alanine side chain have a tendency to associate in an aqueous environment. When water comes in contact with the hydrophobic molecules, then the hydrophobic bond generates. Increased area of contact between water and hydrophobic molecules decreases entropy and higher the energy state (Fig. 5.2d).