In this article we will discuss about Ginkgo. After reading this article you will learn about: 1. Distribution of Ginkgo 2. Nomenclature of Ginkgo 3. Ginkgo: A Living Fossil 4. Morphological Features 5. Anatomy 6. Reproductive Structures 7. Gametophytes 8. Embryogency 9. Economic Importance.
Contents:
- Distribution of Ginkgo
- Nomenclature of Ginkgo
- Ginkgo: A Living Fossil
- Morphological Features of Ginkgo
- Anatomy of Ginkgo
- Reproductive Structures of Ginkgo
- Gametophytes of Ginkgo
- Embryogency of Ginkgo
- Economic Importance of Ginkgo
Contents
1. Distribution of Ginkgo:
Ginkgo biloba is a tall (Fig. 10.3) slender and beautiful tree. It is commonly called Maiden-hair Tree because its new leaves resemble very much like those of Adiantum (called maiden hair fern) both in form and venation.
It is the oldest living seed plant. It is cultivated for its edible seeds in some parts of China and Japan. Though, Chamberlain (1935) mentioned that it is doubtful whether Ginkgo exits today in the wild state, but Sporne (1965) has stated clearly about Ginkgo biloba that “if it occurs naturally anywhere, is restricted to a small and relatively inaccessible region in South China”.
Li (1956), however, mentioned certain evidences that Ginkgo still exists in the wild state in South-eastern China, along “the north western border of Chekiang and south eastern Anhwei”. In China and Japan it is grown as a sacred tree in temple gardens. It is cultivated in the United States as a shade tree. It is also successfully cultivated in some gardens of Europe, America and India.
2. Nomenclature of Ginkgo:
The name ‘Ginkgo’ was first proposed in 1690 by Kaempfer, a European botanist, and the same name was adopted by Linnaeus (1771). Due to the frequently notched character of the fan-shaped leaves, the name “biloba” to the species was, however, proposed by Linnaeus (1771).
According to Moule (1944) and Thommen (1949) the correct spelling should be Ginkyo. Li (1956), however, used and advocated for the word Ginkgo and opined that maiden hair tree has been designated correctly as Ginkgo and “certainly will be known as such forever”. In fact, the tree was formerly known as Salisburia adiantifolia but later the name was changed to Ginkgo biloba.
3. Ginkgo: A Living Fossil:
Ginkgo is known to have occurred in rocks as old as Triassic or even much earlier. Fossils of its leaves have been identified in the Permian and probably also in the Carboniferous. Ginkgo biloba occurs even today. It is, therefore, referred as living fossil by the botanists. Or, it may also be referred as the oldest living seed plant.
In the words of Professor A C. Seward of University of Cambridge, Ginkgo biloba is “an assemblage of changelessness, a heritage from worlds of an age, too remote for our human intelligence to grasp, a tree which has in its keeping the secrets of an immeasurable past”.
4. Morphological Features of Ginkgo:
The plant body of Ginkgo biloba is sporophytic, and the sporophyte resembles several conifers in general habit. The trees have a pronounced ex-current habit of growth and attain a height up to 30 metres. A very irregular pattern of branching is shown by Ginkgo trees.
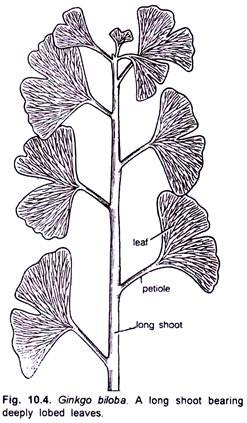
The branches are dimorphic i.e. bear long shoots which are of unlimited growth with scattered leaves and dwarf shoots which are short branches of limited growth.
Long shoots elongate rapidly, sometimes as much as 50 cm in a year. Dwarf shoots grow rather slowly. A dwarf shoot of 2-3 cm length may be several years old. Sometimes there is no clear-cut distinction between two types of shoots, and the dwarf shoot may convert into a long shoot while the latter may sometimes convert into a dwarf shoot for a year or two and then revert back into a long shoot.
The foliage leaves, present on the long shoots, are deeply lobed while those on the dwarf shoots are not so deeply lobed and sometimes more or less entire (Figs. 10.4, 10.5). Bierhorst (1971) opined that differences in the growth pattern of long and dwarf shoots may be due to the quantities of auxin produced in the apical meristems.
Ginkgo biloba possesses a long tap root system. The roots are extensively branched and penetrate deep into the soil. The foliage leaves are simple,large, petiolate and wedge-shaped or fan-shaped with expanded apex and narrow base.
They resemble Maiden-hair fern (Adiantum) and by this they can be distinguished among gymnosperms In general, the leaves are bilobed, and hence the name ‘biloba’ was suggested to the species by Linnaeus (1771).
However, the leaves with many lobes (Fig 10.6) are also present invariably on the tree.The leaves of the long shoot and seedlings are deeply bilobed while that of the dwarf shoot are not so deeply lobed and may be sinuate or even entire. They may be pale yellow, golden yellow or dark green in colour. A typical dichotomous type of venation (Fig. 10.7) is present in the leaves.
Foliar epidermis also exhibits some distinguishable characters in Ginkgo biloba. The epidermal cells are rectangular over the veins while polygonal in outline in between the veins. The leaves are hypostomatic (i.e. bear stomata only on the lower surface of the leaf).
Kanis and Karstens (1963), however, observed a few stomata on the upper surface of the leaves on long shoot, thus making them amphistomatic. Stomata remain surrounded by 4-6 subsidiary cells with finger-like outgrowths overarching the guard cells.
5. Anatomy of Ginkgo:
(i) Root:
In transverse section (Fig. 10.8) the roots are somewhat circular in outline. Mature roots are surrounded by phellogen or suberized cells of cortex. A large portion of the young root is occupied by multilayered, thin-walled cortex which contains several tannin- filled cells and calcium oxalate crystals. Mucilage canals are also prominently visible (Fig. 10.8)
In young roots, a layer of endodermis and uni-layered pericycle are clear. Mature roots,however, lack such a distinction. Diarch condition is clearly visible in the young roots. Xylem is exarch. It remains separated by the phloem strands. Sometimes the roots also show triarch condition.
(ii) Stem:
The young stem (long shoot) is more or less circular in outline and remains surrounded by a single-layered, thickly circularized epidermis (Fig. 10.9) made of brick-shaped cells. Epidermis is replaced by periderm in the older stems.
Inner to the epidermal layer is present a well-marked region of parenchymatous cortex. It contains mucilaginous canals, sphaeraphides and many tannin-filled cells. Cortex is comparatively less extensive in long shoots than dwarf shoots. Endodermis and pericycle are not well-marked in long shoot.
Several conjoint, collateral, open and endarch vascular bundles are arranged in a ring in very young stem. Two leaf traces, one for each leaf, are given out. After the onset of the secondary growth, the vascular cylinder of the stem becomes an endarch siphonostele with no parenchyma in the wood except that of uniseriate medullary rays.
Protoxylem has spiral thickenings while bordered pits are present on the radial walls of the metaxylem tracheids. Sieve tubes and phloem parenchyma constitute the phloem. A narrow pith, containing mucilage canals and sphaeraphides, is present in the centre of long shoot, while in dwarf shoot the pith is comparatively more extensive.
Secondary Growth in Stem:
Cork cambium cuts periderm towards outer side and secondary cortex towards inner side (Fig. 10.10) Periderm replaces the epidermis. Mucilage canals are absent.
A single ring of cambium remains active throughout and cuts secondary phloem towards outer side and secondary xylem towards inner side. Crushed patches of primary phloem towards outer side and primary xylem towards inner side are present. Secondary phloem consists of sieve tubes and phloem parenchyma. Secondary xylem consists of tracheids. Ill-defined annual rings are also seen.
Study of the tangential longitudinal sections of long and dwarf shoots shows that uniseriate medullary’ rays are 1 -3 cells in height in long shoot (Fig. 10.11) while 1-15 cells in height in dwarf shoot (Fig. 10.12).
One or two rows of bordered pits are present on the radial walls of the tracheids (Fig. 10.13). Pits are circular in outline and have a clear torus. Bars of Sanio are also present. Bars of Sanio do not occur in primary wood. Trabeculae of Sanio, which cross the lumen of the tracheids, are also present.
(iii) Leaf:
A layer of epidermis is present on upper as well as lower sides of leaf. The epidermis is thickly cuticularised and consists of rectangular to polygonal cells. Haplocheilic type of stomata, restricted only to the lower epidermis, are present. Kanis and Karstens (1963), however, reported some stomata on the upper epidermis of the leaves on long shoots of male plants only.
Mesophyll is present in between the two epidermal layers. It is not well-differentiated into palisade and spongy parenchyma (Fig. 10.14). The leaves of long shoot, however, show a distinct palisade region. Many mucilage canals or secretory canals and a few tannin-filled cells are also present in the mesophyll region.
Vascular bundles of the vein have an indistinct mesarch structure (Fig. 10.14). Each vascular bundle may be surrounded by a sclerenchymatous bundle sheath in mature leaves.
(iv) Petiole:
The petiole remains covered by a layer of thickly cuticulanzed epidermis, the continuity of which is broken by stomata. Inner to the epidermis are present a few hypodermal layers. Few mucilage canals, tannin-filled cells and sphaeraphides are irregularly distributed in the cortex. The petiole is supplied by a pair of endarch vascular bundles (Fig. 10.15).
The detailed structure of a vascular bundle is shown in Fig. 10.16. It remains surrounded by a sclerenchymatous bundle sheath. Protoxylem has spiral thickenings. Cambium is clearly visible Uniseriate rays are present in the xylem. These are continuous with those of the phloem.
6. Reproductive Structures of Ginkgo:
Ginkgo biloba is dioecious. Male and female plants are,however, difficult to be differentiated, when young. According to Lee (1954) sex in Ginkgo is determined by sex chromosomes (XY in male and XX in female). Reproductive bodies of Ginkgo are most primitive among living seed plants except some Cycadales.
(i) Male Strobilus:
Male or microsporangiate fructifications develop in catkin-like clusters (Fig. 10.17 A) on the dwarf shoots of male frees.Each male strobilus contains several microsporophyll’s arranged loosely on a central axis (Fig. 10.17B). Each microsporophyll has a long stalk terminating into a hump or knob.
It contains two pendant microsporangia (Fig. 10.17C). According to some workers this terminal knob represents an abortive sporangium. A mucilage duct is present in the knob. Rarely more than two sporangia are present in a microsporophyll.
Each sporangium is a tubular structure surrounded by many layers (Fig. 10.17D). The outermost layer of sporangial tissue differentiates into a tapetum. The sporogenous cells of the sporangium undergo reduction division and form many haploid microspores Wolniak (1976) has studied the ultrastructure of the microspore mother cell.
(ii) Development of Microsporangium:
It is of eusporangiate type, i.e., single archesporial cell divides by a periclinal wall forming primary wall cell and primary’ sporogenous cell (Fig. 10.18A,B). The former develops into wall of microsporangium while the latter develops into sporogenous tissue. Sporangium dehisces by means of a longitudinal slit.
(iii) Female Strobilus:
The female strobili develop in groups in the axil of leaves or scaly leaves present on the dwarf shoots (Fig. 10.19). These are much reduced structures, each having a long stalk or peduncle, which bifurcates apically. Each bifurcation usually bears a single ovule. Out of the two sessile ovules one generally aborts earlier.
Rarely, three, four or more ovules on a peduncle are also observed. Sprecher (1907) reported an axis with seven ovules, each borne singly on its independent peduncle, and not in pairs. At the base of each ovule is present a fleshy cup-like structure called ring or collar. The leaves surrounding the ovules do not show their bilobed character.
The development of ovule, mega-sporogenesis and structure of the mature ovule is similar to that of Cycas. There is a thick integument consisting of three layers, i.e., outer fleshy, middle stony and inner fleshy layers (Fig. 10.20). A major difference between Ginkgo and Cycas is that the integuments of Ginkgo do not have any noticeable vascular supply.
Each ovule has a large and prominent nucellus. Free apex of the nucellus breaks down into a pollen chamber (Fig. 10.21) forming the nucellar beak. Quite deep in the nucellus tissue, a functional spore mother cell becomes prominent. The spore mother cell develops into a tetrad, of which only the innermost megaspore remains functional and develops into female gametophyte.
The vascular supply of the ovule is ill-developed. Two vascular strands enter the inner fleshy layer and reach up to the free part of the nucellus without branching. Outer fleshy layer lacks any prominent vascular supply.
7. Gametophytes in Ginkgo:
(i) Male Gametophyte:
Major aspects of the development of male gametophyte of Ginkgo biloba (Fig 10.22) are similar to that of Pinus. Male gametophyte starts developing in situ, i.e. within the sporangium.
Friedman (1987) studied the growth and development of the male gametophyte of Ginkgo biloba using three-dimensional computer reconstruction (Fig. 10.22). Pollen germination starts with a diffuse swelling of tube cell. Tube cell becomes very elaborate with a proximal un-branched tube and a much branched haustorial system.
Microspore is the first cell of the male gametophyte. Each microspore is a rounded structure having thin intine and thick exine layers. A centrally located nucleus contains one or two nucleoli and remains surrounded by dense cytoplasm. An un-thickened portion, called pore, is also present in each microspore. It is a region where exine is not covering the intine.
Two unequal cells are formed by the first mitotic division in the microspore. These are called 1st prothallial cell and inner cell (Fig. 10.23 A). The 1st prothallial cell is smaller than that of inner cell. The inner cell again divides forming a 2nd prothallial cell and an antheridial initial (Fig. 10.23B).
By this time the 1st prothallial cell starts to degenerate. The antheridial initial then divides forming a generative cell near the 2nd prothallial cell and a tube cell (Fig. 10.23D). The tube cell does not divide any further. This is the 4-celled stage of the male gametophyte and the microspores are dispersed by wind at this stage.
Pollination in Ginkgo biloba takes place sometime in April. At the time of pollination the ovule secretes a mucilaginous substance. Further development of the male gametophyte takes place in the pollen chamber. In pollen chamber the generative cell of the 4-celled young male gametophyte divides into a stalk cell, which is close to the 2nd prothallial cell, and a body cell.
Now the intine protrudes out near the pore and functions as a pollen tube (Fig. 10.23E). The pollen rube swells up and advances towards the archegonia by protruding into the nucellus. Just before fertilization the body cells divides into two, and the wall formation is at right angle to the base of pollen grains.
The blepharoplasts appear on the body of these so-formed two cells which function as sperms or male gametes (Fig. 10.23F). The structure of these multi-flagellated sperms, as well as their behaviour in the tube at the time of fertilization has been investigated by Shimamura(1937), Lee (1955) and Favre-Duchartre (1956).
Motile sperms in gymnosperms were observed for the first time on September 9, 1896 by Hirase, and his this discovery is considered as one of the most remarkable events in plant morphology. The ultrastructure of male gamete of Ginkgo has been studied by Gifford and Lin (1975).
(ii) Female Gametophyte:
The development of the ovule and female gametophyte in Ginkgo biloba has been studied by Carothers (1907). Out of the four megaspores formed from the megaspore mother cell, only the lowermost remains functional and the remaining three degenerate (Fig. 10.24) A).
The nucleus of the functional megaspore migrates towards the micropylar end and divides into two followed by a number of free-nuclear divisions forming hundreds of free nuclei.
A large vacuole is present in the megaspore at this stage (Fig 10.24B). Now the wall formation starts (Fig. 10.24B,C) and progresses from the periphery towards the centre. Dunng wall formation first the anticlinal walls are formed followed by the vertical walls.
Each cell generally contains one nucleus but in some cells 2-3 nuclei are also seen. Due to more rapid cell divisions on the micropylar end a pole-like structure develops. This is called tent-pole (Fig. 10.24D). The female gametophyte possesses abundant chlorophyll.
The development of archegonium starts from the cells towards the micropylar end of the female gametophyte. According to Favre-Duchartre (1958) the archegonial initials start appearing sometime in June.
Each archegonium possesses a short neck made up of only four cells and a small venter having a central cell. The central cell later on forms a ventral canal cell and an egg cell (Fig. 10.25). According to Bierhorst (1971) only two cells are present in the archegonial neck.
Ultra structural study of the female gametophyte of Ginkgo biloba has been made by Dexheimer (1973). He observed four zones based on food reserves.
These are:
(1) Lipid zone,
(2) Starch proteolipid zone with large vacuolated cells,
(3) Starch zone made of large vacuolated cells with peripheral cytoplasm, and
(4) Deep or central zone with very little reserve contents.
Fertilization:
The pollen tube reaches up to the neck of the archegonium just after its (archegonium) differentiation. The tube ruptures releasing the sperms and the other contents in the archegonial chamber. A sperm passes through the neck of the archegonium, comes in contact and fuses with the egg nucleus exactly in the same way as in Cycas.
8. Embryogeny in Ginkgo:
The zygote becomes enlarged and starts dividing by many free-nuclear divisions just after fertilization. The nuclei are distributed irregularly (Fig. 10.26A). The cell wall formation starts just after a few divisions of the nucleus and soon a pro-embryo is differentiated (Fig. 10.26B). Three types of cells are clearly differentiated in the pro-embryo (Fig. 10.26C).
The cells towards the chalazal end are smaller in size with dense cytoplasm, followed by another zone of comparatively larger cells, which do not possess such a dense cytoplasm. Elongated cells are present in the uppermost zone. There is no differentiation of suspensor in the embryo.
The development of the cotyledon starts from the lowermost zone soon after the differentiation of these zones (Fig 10.26D,E). Roots, stem and cotyledons are soon differentiated (Fig. 10.26 E,F). Generally, five leaves are present in the mature embryo. Out of these five leaves the first two are decussate with cotyledons while the remaining leaves are irregular in arrangement in embryo and seedling.
The germination of the seed is of hypogeal type and quite similar to that of Cycas. A strong tap root develops soon and the seedling bears many bilobed leaves, which is characteristic feature of Ginkgo biloba. Soon, a mature plant, with many more leaves, develops.
In a recent study, Dogra (1992) opined that development of embryo in Ginkgo biloba is a continuous process from the time of fertilization until the seed germination. Actually, the major part of the growth of embryo takes place when the seed is detached from the tree and is lying on the ground.
9. Economic Importance of Ginkgo:
Ginkgo biloba tree is treated as a sacred plant in China and Japan and worshipped by the people. It is regarded as “Holy Tree” by Buddhist monks and nuns because of its tall, strong and majestic appearance. It is grown as an ornamental plant in gardens in several countries.
In autumn, the green leaves turn yellow, showing a magnificent autumn scenery. The endosperm of its roasted seeds is edible, but seeds may prove fatal if taken in large quantity. After the outer skin of its apricot-like seeds is peeled off or decayed, the kernel left is called ” White Fruit”. Ginkgo is, therefore, also named “White fruit tree”.
The kernel is also used as food and Chinese medicine as well. If it is taken raw, it functions as detoxicating and asthma-curing medicine. Although the plants are highly resistant to pathogenic infections, specially of bacteria and fungi, their wood is not of much commercial importance. It also possesses a strong capacity of resistance to air pollution by smoke and poisonous gases.
Since an unpleasant odour is emitted from the ripe seeds of female trees, male trees are largely preferred and grown as shade trees in China, Japan and United States. Ginkgo trees grow and develop very slowly.
It is said that the tree planted by grandfather will bear “fruit” at the time when his grandson grows up. Therefore, it is also known as “grandfather grandson tree”.An extract of leaves of G. biloba is useful in the treatment of cerebral insufficiency and vertigo.