In this article we will discuss about Ephedra. After reading this article you will learn about: 1. Distribution of Ephedra 2. Vegetative Features of Ephedra 3. Anatomy 4. Reproduction 5. Embryogency 6. Economic Importance.
Contents:
- Distribution of Ephedra
- Vegetative Features of Ephedra
- Anatomy of Ephedra
- Reproduction of Ephedra
- Embryogency of Ephedra
- Economic Importance of Ephedra
Contents
1. Distribution of Ephedra:
Ephedra occurs almost equally in both Western as well as Southern hemispheres. It is distributed in both North and South America, France, Arabia, northern parts of India, China and other neighbouring countries. Out of the so far reported nearly 40 species. The Old World (France, Canary Islands, India, China) accounts for about 18 species whereas 22 species are confined to New World (North and South America).
In India, it is represented by 6 species namely Ephedra foliata, E. gerardiana, E. intermedia, E. nebrodensis, E. regeliana and E saxatilis. They are distributed in dry parts of Punjab, Haryana, Rajasthan, Kashmir and Sikkim. Ephedra foliata is found in drier tracts of plains of Rajasthan, Haryana and Punjab.
2. Vegetative Features of Ephedra:
The vegetative plant body contains roots, stem and leaves and shows xerophytic characters. Shruby plant body usually remains less than two metres in height in most of the species. Ephedra compacta attains a height of about 30 cm and E. triandra, a lone tree species, reaches up to several metres. Chamberlain (1935) mentioned that Ephedra is a short-lived plant. It resembles Equisetum in its external morphology.
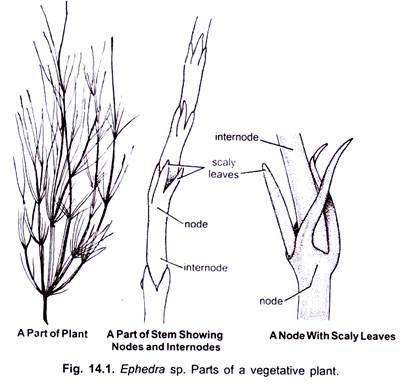
A prominent underground tap root system with many adventitious roots is present in Ephedra. Roots contain many root hairs but there is no mycorrhiza. The stem is green, ribbed, profusely branched, hard and glabrous, and bears nodes and intemodes. It is distinctly jointed. The stem, being green, performs the function of photosynthesis.
The leaves are small, scaly and rudimentary (Fig. 14.1). They are present in opposite decussate manner, or in whorls of three or rarely four. “There is hardly anything which could be called a blade” in Ephedra.
Scaly leaves fuse at the base to form a basal sheath. Each leaf contains two un-branched, parallel veins. A bud, which forms the branch, is present in the axil of each leaf. True foliage leaves are absent in Ephedra.
3. Anatomy of Ephedra:
(i) Young Stem:
The outline shows many ridges and grooves (Fig. 14.2). Epidermis is thickly cuticularized. The epidermal cells are thick-walled. Patches of sclerenchyma are present, particularly below the ridges.
Continuity of the epidermis is broken by many sunken stomata present in the grooves or along the sides of the grooves. The wide zone of thin-walled, chlorophyll-containing green cells is present in between the thick-walled sclerenchyma and the vascular cylinder (Fig. 14.2).
Many intercellular spaces are also present in this region. In some species this green region is differentiated into palisade and spongy parenchyma. Some sclerenchyma patches are also irregularly distributed in this green region. Endodermis is clearly demarcated only in very young stems. Pericycle is not clearly defined.
The vascular cylinder is an endarch siphonostele. It is formed by an interrupted ring of 8- 12 conjoint, collateral, open and endarch vascular bundles. The number of the primary vascular strands is generally eight, out of which four represent the ‘foliar traces’ while the remaining four are ‘stem-bundles’.
Foliar traces run up to the node. Each leaf receives two bundles. The nodes are unilacunar and two-traced type.
In each vascular bundle the xylem is present towards inner side while the phloem towards outer side. The xylem consists of tracheids, vessels and xylem parenchyma. The vessels contain bordered pitted thickenings. The tracheids may be annular or spiral.
The phloem consists of sieve cells, phloem parenchyma and albuminous cells. The albuminous cells look like the companion cells of angiosperms. A well-developed parenchymatous pith is centrally located. It becomes thick and sclerenchymatous in the older stems. Tannin-like contents are present in a group of thick-walled cells in the centre of the pith.
(ii) Old Stem (Showing Secondary Growth):
Vascular cambium, responsible for secondary growth, is present in between primary xylem and primary phloem. After forming a complete ring of cambium, the latter cuts secondary phloem towards outer side and secondary xylem towards inner side (Fig. 14.3). The distinct annual rings are formed because of the varied activity of cambium in different seasons.
The secondary xylem consists of vessels, tracheids and small amount of xylem parenchyma. Fibres are absent. The secondary phloem consists of sieve tubes and companion cells as in Gnetum. These are formed in rows. Some albuminous cells are also present in the secondary phloem. Alosi and Alfieri (1972) grouped sieve cells of Ephedra into two different categories on the basis of their length, i.e. about 220 μ and 400 μ.
The presence of vessels is the most striking anatomical feature of Ephedra. Vessels are modified tracheids of all sizes. The largest vessels are formed in spring but in the later part of the season the size decreases. The bordered pits are present. After some time they become simple perforations by loosing the torus and border of the pits.
In T.L.S. (Fig. 14.4A) medullary rays are clearly demarcated. They are very long, wide and uniseriate, but become multi-senate by the longitudinal divisions. The rays consist of isodiametnc, elongated or curved cells, the size of which varies. Their walls are thick, lignified and pitted. The pits are simple and small. In Ephedra the wood, in general, is hard because most of its cells are thick-walled.
In R.L.S. (Fig. 14.4B) large vessels and many tracheids are seen. The tracheids show bordered pits which are usually uniseriate and more on the radial walls. Vessels are the most prominent features.
The pith becomes narrow in the stems undergoing secondary growth. It becomes very scanty in old-stems.
Carlquist (1992) reported occasional growth rings without vessels in several Old World species of Ephedra. According to him, the first year wood was entirely vessel-less in E. equisetina.
Tiagi (1966) has studied the vascular anatomy of Ephedra foliata while Cressen and Evert (1993) have given an impressive account of the vasculature in the stem of E. viridis.
(iii) Leaf:
In T.S. (Fig. 14.5) the reduced and membranous scaly leaves are somewhat oval in outline. The epidermis consists of elongated or oval cells. Cuticle is also present. Stomata, when present, are sunken. Two to three or more layers of chlorophyll-containing cells of palisade tissue are present.
Major remaining part of the scaly leaf remains filled with cells of spongy parenchyma. Many air spaces are present in the parenchymatous and palisade regions. The vascular bundles are two in number. They are small and remain embedded in the parenchymatous tissue.
4. Reproduction of Ephedra:
Ephedra is dioecious, and the two sex organs are present on different plants. In E.foliata, however, monoecious individuals are also common. Occasionally, an ovulate flower may be replaced by a staminal flower, and thus the strobilus becomes bisporangiate as in E. campylopoda. In the bisporangiate strobili the male flowers are present in the lower region while the female flowers at the top of the strobilus.
Ephedra is heterosporous, i.e., two types of spores (microspores in male flowers and megaspores in the female flowers) are present. The male and female flowers are present in the form of cone-like, compound male and female strobili, respectively.
It is only at this stage that the distinction between the male and female plants can be made because there is no well-marked morphological difference in the vegetative stage of the male and female plants of Ephedra.
Male Strobilus:
The male strobili are compound structures arising in clusters from the nodes of the branches (Fig. 14.6). Each strobilus develops in the axil of a scaly leaf (bract).
A male strobilus is a round or ovoid body (Fig. 14.7) with a strobilus axis in its centre. Two to eight pairs of bracts remain arranged in opposite decussate manner on the strobilus axis (Fig. 14.8). All the bracts are fertile except a few on the lower side. A single male or staminate flower arises in the axil of each bract (Fig. 14.9).
Each male flower consists of two bracteoles and a stamen. Bracteoles are thin opposite scales united at the base. They have been interpreted as perianth. Each stamen is a stalked structure.
The stalk continues into a short axis or micro-sporangiophore which bears two to eight or more microsporangia terminally. Microsporangia are sessile structures. Each microsporangium is bilocular or trilocular. The male flower is called a simple strobilus.
Development of Microsporangium:
The microsporangium starts to develop (Fig. 14.10) from a group of hypodermal cells which function as archesporium. The archesporial cells are larger in size with more dense cytoplasm and quite prominent nuclei in comparison with the other adjacent cells. These cells divide periclinally into outer primary parietal cells and inner primary sporogenous cells.
The parietal cells divide repeatedly and form one-celled thick wall and also the tapetum. But according to Singh and Maheshwari (1962) the primary wall layer functions directly as the outermost wall of the sporangium while the primary sporogenous cells from a middle wall layer, an inner tapetal layer and sporogenous cells.
The sporogenous cells divide several times irregularly and form many microspore mother cells. The latter divide meiotically to form spore tetrads arranged tetrahedrally. The haploid microspores are later on separated. Rarely, the spores are arranged iso-bilaterally.
Land (1904,1907) observed that one layer of crushed wall cells is present in between the outermost layer and tapetum layer of sporangium. The tapetum cells are very large.
Female Strobilus:
The ovulate or female strobili are elongated and pointed structures (Figs. 14.11,14.12). Similar to male strobili they also develop in the axil of leaves in the whorls of 2, 3 or 4 at the nodes of small green branches. Each female strobilus is sessile and smaller than male strobilus. Pairs of bracts are more in number in female strobilus than male strobilus Bracts are arranged in opposite decussate manner.
All bracts, except the uppermost pair, are sterile. Two ovules are present in the axil of uppermost pair of bracts, out of which generally only one survives. The female strobili appear on the plants in the month of April and the mature seeds are seen in September in Ephedra gerardiana.
Ovule:
The ovule remains covered by a cup-shaped outer integument and an inner integument. The outer integument remains attached at the basal portion of the ovule. The inner integument protrudes out in the form of a long tubular micropyle. The integuments enclose the nucellus.
A small pollen chamber develops near the micropyle in the nucellus. Pollen chamber in Ephedra is deepest known among the Gymnosperms. The female gametophyte is centrally located (Fig. 14.13), and the archegonia are present in the female gametophyte near the micropylar end. The haustorial region is present near the chalazal end and bears some haustorial processes.
A hypodermal archesporial cell gets differentiated in the parenchymatous nucellus of the young ovule. This archesporial cell divides periclinally into outer parietal cell and an inner megaspore mother cell. The megaspore mother cell is pushed quite deep into the nucellar tissue and divides meiotically to form four haploid megaspores.
The archesporial cell functions directly as a megaspore mother cell in Ephedra inter-media. T-shaped megaspore tetrads have been observed in Ephedra foliata by Maheshwari (1935) Generally the lowermost megaspore, situated near the chalazal end, remains functional and develops into the female gametophyte.
Micro-Sporogenesis and Male Gametophyte:
The microspore is the first cell of the male gametophyte. It is wingless, inaperturate and has a thick exine. The germination of microspore starts within the microsporangium. At the time of germination, the microspore elongates and divides to form a prothallial cell (Fig. 14.14 A).
The second division results in the formation of a 2nd prothallial cell (Fig. 14.14B). In Ephedra trifurca this second prothallial cell is not separated from the antheridial initial by a wall (Land, 1904) and the same condition has been reported by Maheshwari (1935) in Ephedra foliata.
But according to Mehra (1938) a wall is present around the second prothallial nucleus in E. gerardina and E. saxatilis but it soon breaks down. The antheridial initial soon divides into a tube cell and a generative cell (Fig. 14.14C, D).
The generative cell soon divides into the nuclei of stalk cell and body cell (Fig. 14.14E, F). A common mass of cytoplasm surrounds the nuclei of stalk cell and body cell and they are never separated by a cell wall (Land, 1904). Pollens are shed at this five-celled stage and are carried up to the micropyle of ovule with the help of wind.
Upon reaching the ovule, the exine of the pollen grains ruptures and the intine comes out in the form of a tube. The generative cell divides and forms two male nuclei (Fig. 14.15).
Mehra (1947) reported diploid pollen grains in Ephedra altissima, E. foliata, E. intermedia and E. saxatilis.
Female Gametophyte:
The functional megaspore is the first cell of the female gametophyte. It enlarges and its nucleus divides several times by many free-nuclear divisions to form as many as 256 nuclei in Ephedra trifurca and 500 nuclei in E. foliata.
The free-nuclear divisions continue for about twenty days. The nuclei get themselves arranged around the central vacuole on the periphery of the megaspore. The free-nuclei are evenly distributed throughout. The wall formation starts from outside, proceeds rapidly towards the centre making the complete structure ultimately cellular.
In this cellular female gametophyte, the cells of the upper reproductive region are large and elongated as compared to that of the lower nutritive region. The nutritive region gets further differentiated into upper storage region and lower haustorium. Archegonia develop in the micropylar region.
Archegonium:
The number of archegonia in Ephedra varies from 1 -3 but their usual number is 2. An archegonial initial divides periclinaly to form an upper primary neck cell and an inner central cell (Fig. 14.17A). The primary neck cell divides several times to form 4-5 or more tiers and then appear anticlinal divisions.
More than 32 neck cells are formed after anticlinal divisions (Fig. 14.17 B, C). Due to certain irregular divisions in neck cells, its tissue sometimes becomes undistinguishable from the surrounding cells of the female gametophyte. The archegonial neck in Ephedra may sometimes become as long as 40 cells.
The central cell enlarges (Fig. 14.17B) and its nucleus divides into a ventral canal nucleus and an egg nucleus (Fig. 14.17C). There is no wall formation between these two nuclei. The ventral canal nucleus may or may not move down towards the egg nucleus. The cells adjacent to central cell may divide transversely to form a clear 2-3 layered jacket.
A mature archegonium (Fig. 14.17C) thus consists of a long multilayered neck and a central cell containing a ventral canal nucleus and an egg nucleus. The long columnar neck appears similar to the transmitting tissue in the style of angiosperms. The archegonium in Ephedra is rich in protein but poor in polysaccharides and RNA. The mature egg is Feulgen negative.
Fertilization:
At the time of fertilization, the pollen tube penetrates the archegonium and discharges its contents into the egg cytoplasm (Fig. 14.18). One of the two male nuclei fuses with the egg nucleus and forms an oospore or zygote. The other male nucleus may fuse with the ventral canal nucleus exhibiting the phenomenon of ‘double fertilization’.
This second fusion, however, does not result into any embryo formation (Khan, 1943). Chamberlain (1935), however, stated that “whether there is any ‘double fertilization’ like that in angiosperms, is questionable”.
Land (1907), however, opined that there is no definite fusion of the second male nucleus with the ventral canal nucleus in Ephedra. But Herzfeld (1922) observed that there is a clear cut double fertilization in E.campylopoda as in angiosperms.
Regarding the double fertilization in Ephedra Khan’s (1943) view may be quoted that “the type of double-fertilization seen in Ephedra may have no phylogenetic significance at all and may simply be the natural outcome of a tendency towards fusion between any two nuclei of opposite sexual potencies that happen to lie free in common chamber”.
Friedman (1990,1991,1992) reported a regular occurrence of double fertilization in Ephedra trifurca and E. nevadensis. According to him double fertilization first evolved in a common ancestor of Gnetales and angiosperms.
The product of second fertilization was diploid giving rise to a supernumerary embryo as observed in Ephedra. This supernumerary embryo later gave rise to an embryo nourishing structure or the endosperms as seen in angiosperms.
5. Embryogeny:
The zygotic nucleus undergoes free-nuclear divisions resulting into eight free-nuclei which remain evenly distributed throughout the cytoplasm. A cell gets organised around each of these nuclei, and each of such cell functions as a potential pro-embryo. Khan (1943) observed that six out of these eight nuclei get surrounded by globular cells and function as six potential embryos.
Such a stage represents polyembryony without any cleavage, and Ephedra is unique among gymnosperms to show such type of polyembryony. A few of such pro-embryos show initial development but ultimately only one survives. The ripe seed thus normally contains only one embryo.
A tubular outgrowth, called suspensor tube, develops from the pro-embryo. Its nucleus also divides simultaneously into two nuclei i.e., embryo nucleus and suspensor nucleus. The wall between the two nuclei is formed.
The embryo nucleus passes into the tube which develops continuously and carries the lower embryonal cell deep into the female pro-thallus. The embryonal cell divides and develops into the embryo-proper which contains two cotyledons.
Electron microscopic studies of Ephedra made by Moussel (1978) suggest that cytoplasmic organelles of the coenocytic pro-embryo are derived mainly from the egg only i.e., they are maternal in origin. In most other gymnosperms, however, the inheritance is paternal in origin.
Seed:
The seed contains a dicotyledonous embryo. It remains situated at the tip of the elongated suspensor and remains embedded within the tissue of the female gametophyte. The remnants of nucellus are seen in the form of a dis-organised sheath of cells.
The seed coat consists of two separate layers derived from two layers of the envelope. At maturity the subtending adjacent bracts of the strobilus in Ephedra foliata become thick and fleshy and form an additional covering over the seed (Fig. 14.20). Thus the seed remains covered by three envelopes.
Seed Germination:
The seed germinates without any resting period and the germination is of epigeal type. Sometimes the seed may even germinate within the parent strobilus. The cotyledons grow steadily until they become several centimeters long.
Cytology:
Species of Ephedra are either diploid (2n= 14) or tetraploid (2n=28). Rarely the number may be 30.
6. Economic Importance of Ephedra:
Besides some ornamental species, an antibiotic, ‘ephedrine’ is obtained from several species of Ephedra, such as E gerardiana, E. intermedia and E. nebrodensis. Ephedrine is effective in treating asthma, bronchitis, cough and hay-fever.
In Russia a decoction, prepared from the roots and stem of several species of this genus, is used in the treatment of syphilis and rheumatism. Tincture of E. gerardiana is effective as a cardiac and circulatory stimulant.