The following points highlight the two main types of screening techniques. The types are: 1. Colony Hybridisation Technique 2. Blotting Techniques.
Type # 1. Colony Hybridisation Technique:
This technique has been developed by Gaustein and Hogness (1975). It requires the radioactively labelled DNA probe with a sequence complementary to at-least one part of the desired DNA.
The probe may be partially pure mRNA, a chemically synthesized oligonucleotide or a related gene, which identifies the corresponding recombinant DNA. Colonies to be tested are replicated from a solid medium on to a nitrocellulose filter paper.
A portion of each colony remains on the medium, which is called master plate. The paper is treated with NaOH, which simultaneously breaks open the cells and denatures the DNA.
The paper is then saturated with labelled mRNA, complementary to the gene being sought, and DNA-RNA renaturation occurs. After washing to remove unbound (32p) mRNA, the position of the bound radioactive phosphorus usually detected by autoradiography, desired colonies are located (Fig. 22.9).
If the protein product of a gene of interest is synthesized, immunological techniques allow the protein producing colonies to be identified using radioactive labelled antibodies.
Type # 2. Blotting Techniques:
A mixture of DNA, RNA or protein fragments of different molecular weight can be separated by gel-electrophoresis due to their different mobility and can be visualized directly in gel. But to confirm the identity of these bands with a known and available molecular probe, it is possible to hybridize these bands with a labelled probe.
When the DNA bands are thus identified by blotting, it is called Southern blotting; when RNA bands are identified, it is called Northern blotting; and when the protein bands are detected, the technique is called the Western blotting.
(a) Southern Blotting Technique:
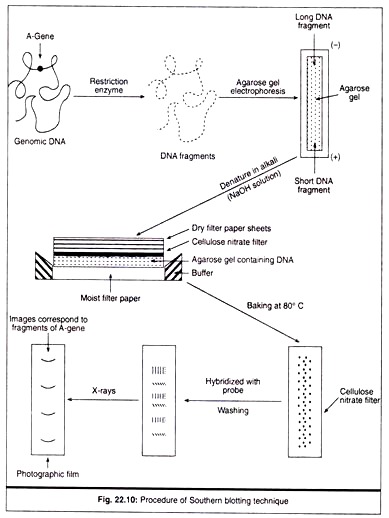
This method was developed by Southern (1975) for analysing the related genes in DNA restriction fragment. This technique helps to provide physical map of restriction sites within a gene located normally on a chromosome, reveals the number of copies of the gene in the genome, the degree of similarity of the gene when compared with other complementary genes.
In the method, the genomic DNA is digested with one or many restriction enzymes and the digested sample is gel-electrophoresed, consequently the DNA fragments are then denatured by alkali treatment; the gel is then laid on top of a buffer saturated filter paper, placed on a solid support; upper surface of the gel is covered with nitrocellulose filter paper overlaid with dry filter paper and then stack of paper-towel.
The dry filter paper when draws the buffer through the gel, the single stranded DNA molecules get attached to the nitrocellulose membrane; after baking at 80°C, DNA fragments are permanently fixed to the membrane; the filter is then allowed to hybridize with radio-labelled probe (DNA/RNA) of known sequence which is complementary to blot- transferred DNA.
After hybridization the filter is washed thoroughly in buffer; the hybridized band regions are detected auto-radio-graphically by placing the nitrocellulose filter in contact with photographic film. The images showed the hybridized DNA molecules (Fig. 22.10).
(b) Northern Blotting Technique:
Alwine et al. (1979) devised the technique in which RNA bands are blot-transferred from the gel onto chemically reactive paper (amino-benzyl oxy-methyl cellulose). The hybridized bands are found out by autoradiography following the same method like Southern. But the name Northern blotting came as it is the extended method of Southern.
In this technique the blot-transfers are reusable because of the firm covalent bonding of RNA to the reactive paper. Thomas invented the technique that under appropriate condition the mRNA can also be blot-transferred to nitrocellulose paper and are then hybridized by single stranded probe (RNA or DNA). The hybrids are treated with SI nuclease to remove the single stranded non-hybridized probe, which helps in quantitative estimation of mRNA.
(c) Western Blotting Technique:
Towbin et al. (1979) developed the technique to detect the proteins of a particular specificity. When the transferred gene expresses the protein in alien cell, then it is detected through this technique.
The technique follows the steps: electrophoresis of cell extract (protein) in polyacrylamide gel; blotting of proteins on to nitrocellulose filter paper; hybridization of proteins by using radiolabelled antibodies (I125antibodies) of known structure; and detection of hybridized sequences by autoradiography. If the radioactive label is not used, the bound antibody may be detected by a second antibody tagged with an enzyme.
(d) Dot Blot or Slot Blot Technique:
In this technique pure extracted genomic DNA to be tested are spotted on a nitrocellulose membrane, where an apparatus is used for placing spots on the membrane through slots, thus the spots are made in the form of oblong slots rather than round blots.
There is no step involving digestion with enzyme; gel electrophoresis or transfer from gel to membrane is needed. This technique is considered much more convenient, when no restriction sites are needed to be studied, only to detect the presence of foreign DNA clone in the cell/tissue.