The below mentioned article provides an overview on Prions. After reading this article you will learn about: 1. History of Prions 2. Structure of Prions 3. Multiplication 4. Diseases.
Contents:
- History of Prions
- Structure of Prions
- Multiplication of Prions
- Prion Diseases
1. History of Prions:
Prion is an acronym for ‘proteinaceous infectious particle.’ In the mid-1960s, Tikvah Alper and co-workers reported that nucleic acid was unlikely to be a component of the infectious agent that causes scrapie disease. In 1967, J. S. Griffith speculated that the scrapie agent might be a protein capable of ‘self-replication’ without nucleic acid.
However, Stanley B. Prusiner, a Neurologist at the University of California at San Francisco, was the first in the early 1980’s to successfully purify the infectious agent and to show that it consists mostly of proteins (technically, it is a glycoprotein, because it has a sugar group attached). He coined the term prion in 1982 for the new pathogen consisting solely of protein, responsible for neurodegenerative diseases called Transmissible Spongiform Encephalopathies (TSEs).
These include scrapie disease in sheep, bovine spongiform encephalopath (BSE or made cow disease) in cattle, Creutzfeldt-Jakob Disease (CJD) and Kuru in humans. He chose this name to distinguish this new pathogen from virus and viroid’s.
Prusiner was awarded the noble prize in medicine for a revolutionary theory ‘the prion hypothesis. In 2007 biochemist Surachai Supattapon and co-workers produced purified infectious prions (PrPc co-purified lipids, and a synthetic polyanionic molecule). In 2010 Jiyan. Ma and co-workers purified infectious prions. In 2011, it was demonstrated that prions can be degraded by lichens.
Prions are something unique existing somewhere in the border zone between living things and non-living matter. Prion, an abnormal from of normal cell protein (PrP) accumulate in brain and progressively damage and destroy brain cells. Prion protein can be transmitted in other individuals of the same or closely related species, by injection or ingestion of infected tissue and appear to be transmissible between species that are not closely related e.g., between cattle and humans.
Proteins showing prion type behaviour are also found in some fungi. As of 2010, there are 8 known prion proteins in fungi (7 in Saccharomyces cerevisae and 1 is in Podospora anserina). These has been useful in helping to understand mammalian prions. Fungal prions do not appear to cause disease in hosts (non-lethal prions).
2. Structure of Prions:
The prions are exclusively composed of sialogycoprotein called prion protein (PrP). They contain no nucleic acid. This protein is found throughout the body even in healthy people and animals. However, PrP found in infections particles has a different structure and is resistant to proteases, the enzyme in the body that can normally break down proteins.
The normal form of protein is PrPc while the infections form is called Pr Psc (the c refers to cellular or common PrP while the sc refer to scrapie, a prion disease occurring in sheep). PrPc is a normal protein found in the membrane of cells.
It has 209 amino acids (in humans), one disulfide bond, a molecular mass of 35-36 KDa and a mainly alpha-helical structure. The infectious isoform of PrP known as PrPSc, is able to convert normal PrPc proteins into infectious isoform by changing their conformation, or shape, this in turn, alters the way, the prion interconnect. Although the exact 3D structure of PrPSc is not known it has a higher proportion of B-sheet structure in place of the normal a-helix structure.
3. Multiplication of Prions:
Prions multiply by transmitting a misfold protein state. When prion enters a healthy organism, it induces existing properly folded proteins to into disease associated prion form. The prion acts as a template to guide misfolding of more proteins into prion form. These newly formed prions can then go on to convert more proteins themselves, this triggers a chain of reaction that produces a large amount of the prion forms. To-explain this Heterodimer model is given.
Heterodimer Model:
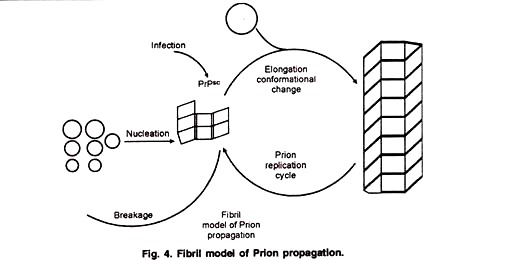
This model tries to explain how prions replicate in a protein only manner. Single PrPsc molecule binds to a single PrPc molecule and catalyzes its conversion into PrPsc. The two PrPsc molecules then come apart and can go on to convert more PrPc (Fig. 3). An alternative model assumes that PrPsc exists only as fibrils, and that fibril ends bind PrPc and convert it into PrPsc (Fig. 4).
4. Prion Diseases:
Prion diseases (also called transmissible spongiform encephalopathy) are very rare: All most all known prion diseases are neurologic diseases. Creutz Feldt-Jacob disease, make up about 85% of the cases. There are about 1 to 1.5 cases per one million people per year.
Human Prion Diseases:
Creutzfeldt-Jacob Disease (Bovine Spongiform Encephalopathy):
A person can get the disease by eating of BSE (Bovine spongiform encephalopathy) infected beef having a blood transfusion, from a person with the disease, injection of human growth hormone extracted from bodies with the disease, or through infected surgical instruments.
Patients suffer from Ataxia or disequilibrium (a patient cannot stand or walk well because he cannot maintain his equilibrium. This is because of a disease in cerebellum), dementia or loss of mentality (progressive loss of cognitive functions) usually die after one year.
Kuru Disease:
This disease is seen people living in New Guinea who eat the brains of dead people. Patients suffer from ataxia, dementia and inability in moving their eyes. Patients usually die within two years.
Gerstmann-Straussler-Scheinker Syndrome:
Very rare syndrome, presents with ataxia and dementia Animal prion diseases.
Bovine Spongiform Encephalopathy (BSE).
Feline Spongiform Encephalopathy.
Ungulate Spongiform Encephalopathy.
Chronic Wasting Disease (CWD)
Scrapie.