The below mentioned article provides notes on DNA replication in prokaryotic and eukaryotic organisms.
Notes # Meaning of DNA Replication:
A double-stranded DNA molecule is capable of producing two identical molecules from nucleotide monomers with the help of some proteins. This is known as DNA replication. DNA needs replication, because every cell produced by division of a pre-existing cell must be provided with an identical genetic material. This is achieved by the semi-conservative mechanism of DNA replication in which each of the two parental DNA strands is used as template for synthesizing two new complementary strands.
The complementarity is based on pairing between A and T, and between G and C. The nucleotides are added one by one from the precursors maintaining the complementarity, to the growing polynucleotide chain using the mother template strand which remains intact. The addition of every new nucleotide to the polynucleotide chain is a step in the process of polymerization. The rate of polymerization in DNA replication is very fast. For example in bacteria, it may be up to 500 nucleotides per second. In eukaryotes, it is nearly 10-fold slower.
The addition of a nucleotide monomer to an elongating polynucleotide chain is shown in Fig. 9.14:
Notes # The Replication Fork:
Experimental observations of replicating DNA suggested that replication involved a localized area of the DNA molecule which moved along the parental helix. The Y-shaped area is known as the replication fork. The two arms of the Y-shaped fork represent the separated strands of the parental helix and the complementary strands of the daughter helices, while the stem of Y represents the un-separated strands of the parental helix (Fig. 9.15).
Replication fork is an asymmetric structure, because the replication in the two arms of Y is not similar. The two strands of a DNA helix have opposite polarity (antiparallel) and DNA replication through polymerization can proceed only in one direction (5′ —> 3′), because the enzyme DNA polymerase can add the incoming nucleotide only to the 3′-OH group of the last nucleotide of the elongating polynucleotide chain.
Thus, DNA polymerization can proceed continuously along one of the two strands used as template. This is called the leading strand. When the other strand is used as template, DNA synthesis proceeds in short pieces also in 5′ —> 3′ direction.
These pieces are known as Okazaki fragments. The 3′ —> 5′ strand of the parental helix is called the lagging strand. The Okazaki fragments are later joined to make a continuous strand. This is diagrammatically represented in Fig. 9.16. The Okazaki fragments of eukaryotic organisms are about 100-200 base long and in prokaryotes about 1,000 to 2,000 base long.
Notes # DNA-Polymerases:
DNA replication is catalysed by the enzyme, DNA-polymerase. In bacteria, like E. coli, there are three different DNA-polymerases, — I, II and III. Of these, DNA polymerase III (pol III) catalyses addition of nucleotides at the 3′-OH end of the elongating polynucleotide chain. This enzyme (pol III) is specific for the precursors used in DNA replication which are 5′-nucleoside triphosphates. From these precursors, pyrophosphate is released after transfer of the nucleoside monophosphate to the acceptor (polynucleotide chain). Pyrophosphate is hydrolysed to release enough energy required for effecting the step of polymerization.
The mechanism involved in chain elongation catalysed by DNA-polymerase is shown in Fig. 9.17. catalyses the addition of mononucleotide units to the free 3′-OH end of a DNA chain. The 3′-OH group at the growing end of the chain attacks the a-phosphorus atom of the incoming nucleoside triphosphate displacing the pyrophosphate group and forming an inter-nucleotide linkage.
Although DNA polymerase catalyses chain elongation, the enzyme is unable to initiate DNA synthesis by linking two nucleoside phosphates together. The enzyme obligately requires a 3′-OH end of a base-paired primer strand to which it can add nucleotides and continue elongating it. Interestingly, such primers are invariably short segments of RNA which form a complementary strand to the template DNA strand.
For the leading strand such an RNA primer is required only at the initiation of the DNA strand synthesis. But for the lagging strand, the primer is required for initiation of each Okazaki fragment. Thus, initiation of DNA synthesis always requires an RNA primer and the first deoxynucleotide is added to the 3′-OH group of the primer. The 5′-end of the primer contains three phosphate groups.
RNA primers for DNA synthesis are synthesized not by RNA-polymerase, as most RNA’s are, but by a different enzyme, known as RNA-primase. When Okazaki fragments are joined to make a continuous strand, the RNA primers are removed by excision and the gaps are filled by new DNA synthesis at the 3′-OH groups of Okazaki fragments and joined by ligase.
DNA synthesis on leading and lagging template strands are diagrammatically shown in Fig. 9.18:
Notes # Other Enzymes and Proteins Required for DNA Replication:
Although DNA-polymerase is the main enzyme involved in DNA-replication, several other enzymes and proteins are also essential. These are necessary to unwind the double helix by disrupting the H-bonds and opening the single-strands, as well as to keep the single strands in proper condition by preventing intra-strand H-bond formation.
These enzymes and other proteins with their function in DNA replication are shown in Table 9.1:
To make the DNA polymerase accessible to the template strand — so that it may effectively bind to it, as well as to facilitate access of incoming precursor to base-pair with the template — it becomes essential to unwind and open the double-stranded DNA helix.
This is accomplished by the enzyme DNA-helicase. There are two different helicases, one for the leading strand and the other for the lagging strand. Both these helicases require ATP hydrolysis for their activity. These enzymes move along a DNA strand and they unwind and open the double helix ahead of the replication fork.
Another group of proteins, called single-strand binding proteins (SSB proteins), bind to the exposed single-strand DNA templates without covering the bases, so that they may be freely available for base pairing with the incoming precursors. These proteins serve to prevent formation of short hairpin double helices by intra-strand H-bonding which might otherwise interfere with effective base- pairing between the template and the incoming precursors.
The functions of helicases and SSB-proteins are schematically represented in Fig. 9.19:
RNA primase which synthesizes RNA primer for initiation of DNA polynucleotide chain, remains associated with DNA helicase to form a composite unit known as primo-some. Still another class of enzymes, known as topoisomerases. Unwinding of the DNA double helix leads necessarily to over-winding of the un-replicated portion. Such over-winding could be relieved by rotation of the overwound portion in the opposite direction.
Due to the enormous length of the DNA molecule, such rotation is not practically feasible. An alternative is to cut open one or both strands of the helix to relieve the tension and rejoin them. These are achieved by topoisomerase I and II enzymes.
The DNA-polymerase has a natural tendency to synthesise short stretches of polynucleotide strand and then leave the template. Such behaviour is suitable for replication of the lagging strand, because after synthesis of one Okazaki fragment, the polymerase may leave the template and initiate synthesis of another fragment.
However, it is unsuitable for the leading strand. In the latter, a long stretch of DNA is required to be synthesized and, therefore, the tendency of the polymerase to fall off the template needs to be prevented. This is managed by the clamp-protein which is a ring-formed molecule. The clamp-protein is attached at the back of the DNA-polymerase and keeps it in place and helps it to slide along the template, so that a long stretch of DNA can be synthesized.
A schematic representation is shown in Fig. 9.20:
Notes # Origin of Replication:
Replication of ds-DNA of both prokaryotes and eukaryotes, as well as of some viruses, is initiated at a unique sequence, called the replication origin (ori). The sequence is different in different organisms and may be up to 300 nucleotide long. In E. coli, the replication origin is a 245 bp sequence.
A local melting of the double helix of this sequence leads to the formation of a replication bubble. The two separated strands then act as template for DNA synthesis proceeding in opposite directions as shown in Fig. 9.21.
The advancing forks move in opposite directions until the whole DNA molecule has been replicated to produce two identical daughter molecules. The initiation of DNA replication requires a complex of several proteins, called the initiation proteins. However, the interaction of the initiation proteins with the ds-DNA is more complex.
Notes # Replication of Circular DNA Molecules:
Circular DNA molecules occur in both prokaryotes and eukaryotes. In prokaryotic organisms, the genome is universally circular. Also, the extra-chromosomal genetic elements, called plasmids are circular DNA. In eukaryotic organisms, circular ds-DNA occurs in the mitochondria and chloroplasts with few exceptions, like Chlamydomonas and some protozoa.
Replication of E. coli chromosome may be treated as a model for replication of circular ds-DNA. E. coli chromosome is a huge double-stranded covalently closed circular DNA molecule which replicates as a circle i.e. its circularity is maintained throughout the replication process. Its replication, therefore, results in the formation of two separate circular chromosomes.
Replication of the circular molecule begins at the origin sequence which binds to the initiation proteins by wrapping the ds-DNA around them to form a protein-DNA complex. This complex then binds DNA-helicase and transfers it to an exposed single-strand of DNA. RNA-primase then binds to the helicase forming a primo some.
The primo some synthesizes the RNA primer. With movement of the primo some, DNA strands separate and opens the template for attachment of the DNA-polymerase. DNA synthesis starts by addition of nucleotides to the RNA-primer through the action of the polymerase.
Initiation of DNA synthesis starts at a bubble produced by disruption of H-bonds between the two strands of DNA. In the bubble, synthesis in the leading strand goes ahead in comparison to the lagging strand. Thus, at the beginning, the bubble consists of a single strand (lagging strand) and a double stranded branch.
These events are diagrammatically shown in Fig. 9.22:
The growing leading strand displaces the lagging strand of the parental template forming a loop which is generally called the D-loop (displacement loop). The synthesis against the lagging strand is also soon initiated. D-loop formation is shown in Fig. 9.23.
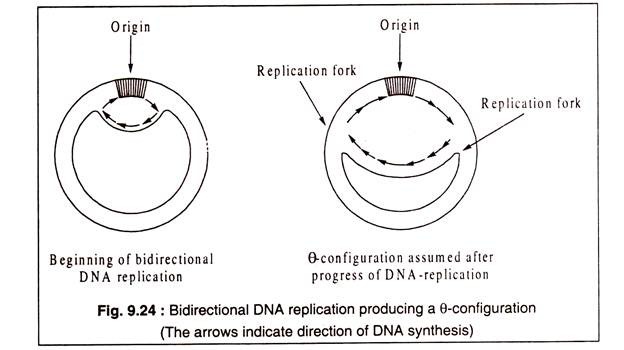
Except in some rare cases, e.g. some phages and plasmids, DNA replication in bacteria, plasmids and eukaryotic organisms is bidirectional which means that two replication forks are produced which move in opposite directions from the origin, one clockwise and the other anticlockwise (Fig. 9.24). The replicating circular DNA forms typically a θ (theta) configuration.
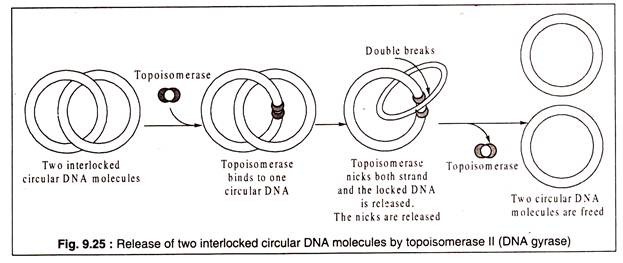
When a ds-circular DNA replicates, the two resulting circular ds-DNA molecules are often locked to each other like the links of a chain to form a catenane. The locked DNA molecules are separated by DNA-gyrase (topoisomerase II). The enzyme cuts both strands of one of the circles and passes the other through the opening.
The nicked ends are then resealed (Fig. 9.25):
Notes # Rolling Circle Replication:
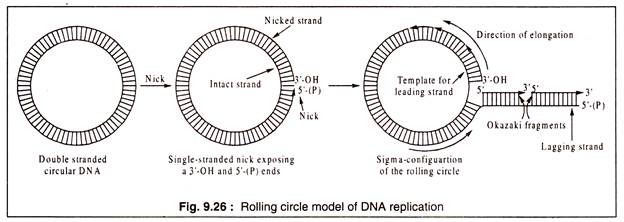
In many bacteriophages and also in bacterial conjugation, a circular double-stranded DNA molecule gives rise to a linear DNA by rolling circle replication. In this type of DNA-replication, a break (nick) is produced in one strand to expose a 3′-OH end and a 5′-(P) end. A replication fork is produced by a helicase and associated SSB proteins.
The 5′-(P) end is displaced and it acts as the template for synthesis of the lagging strand in Okazaki fragments in the 5’—>3′ direction. The exposed 3′-OH end of the nicked strand can add nucleotides from precursors to elongate the polynucleotide chain using the intact DNA strand as template. Rolling circle implication results in a sigma (a) configuration consisting of a rolling circle and a linear branch (Fig. 9.26).
Rolling circle replication occurs in X-phage which has a linear ds-DNA genome in the virions. After the phage enters into the host E. coli the linear molecule circularizes with its cohesive ends (cos) to form a circular ds-DNA.
Towards the end of the infection cycle, this circular molecule replicates by rolling circle process to produce a long linear ds-DNA in which the phage genome is repeated several times. Ultimately, during packaging in the phage heads, the long DNA molecule is cleaved to form the genomic DNA.
Rolling circle replication also occurs in F-plasmids of conjugating bacteria, like E. coli. The F-plasmid which confers the ability to conjugate is a circular ds-DNA. During conjugation, the F- plasmid replicates by rolling circle process to yield a single-stranded copy which is passed into a recipient (F–) cell.
The copy becomes double-stranded by synthesizing a complementary strand. The F- plasmid of the donor (F+) also becomes double-stranded. Rolling circle replication occurs also in single-stranded DNA phages, like φX174 which reproduces through a double-stranded intermediate.