The below mentioned article provides a quick notes on Isolation of Protoplasts and Protoplast Culture.
Klerker (1892) made unsuccessful attempts to isolate protoplasts. In 1909 Kunster isolated protoplasts by mechanical method. Effective technique of protoplast isolation was evolved by Cocking (’60), Nagata and Takebe (’70) and Bhojwani and Cocking (’72). Enzymes used for isolation of protoplasts are cellulase, hemicellulase and pectinase.
Early works of protoplast isolation were done with sponey and palisade cells from the leaves of Nicotiana and Petunia. But later protoplasts from various tissues, such as, callus of Gossypium hirsutum and Solanum tuberosum, anthers of Pelargonium etc., were cultured.
P. S. Rao (’84) obtained a complete plant by culturing protoplasts isolated from the tissue of sandlewood tree (Santalum album) (Plate I and II). This is the first report of successful development of a whole plant from the protoplast of a tree.
Method:
Before removal of the cell wall the cells should be placed in an isotonic plasmolyticum, such as 13% mannitol or sorbitol. Some scientists used low osmotic potential with 0.2M sucrose or 2% polyvenylpyrrilodine. According to P.K. Evans and E. C. Cocking .very low concentration may result in fusion of protoplasts producing multinucleate protoplasts.
Protoplasts can be isolated from the cells by two methods:
(a) Mechanical method,
(b) Enzymatic digestion of the cell wall.
(a) Mechanical method:
The cell walls of plasmolysed cells are cut mechanically. During this condition the protoplast remains in a contracted state. On subsequent deplasmolysis the protoplast swells and comes out of the cell wall. This method is not convenient and only a small percentage of protoplast remains viable. But this method is devoid of any harmful action caused by the enzymatic process.
(b) Enzymatic method:
This method is used since early sixties. This method consists of:
(i) Surface sterilization of leaves,
(ii) Rinsed in proper osmoticum,
(iii) Lower epidermis is peeled off or the material is sliced to help entry of the enzymatic solution,
(iv) Treated with the enzymatic solution,
(v) Removal of cell debris or enzymes and purification of the protoplasts,
(vi) Protoplasts are transferred to a suitable culture medium.
The cell wall is mainly composed of cellulose, hemictllulose, pectin, some amount of lipid and protein. Cellulose is a polymer of glucose. Xylan is a kind of hemicellulose. Pectins are polysaccharides containing sugars, such as, galactose, arabinose, galactouronic acid—a derivative of galactose. Adjacent cells are attached to one another by the middle lamella, which is a pectic substance.
Enzymes used for cell wall degradation are cellulase, hemicellulase and pectinase.
Enzyme treatment may be done in two ways:
(a) In mixed-enzyme method cellulase, hemicellulase and pectinase mixture is used for cell wall degradation,
(b) In sequential method first pectinase is used. This will separate the cells. Then cellulase, hemicellulase mixture is used for digestion of the cell wall.
Usually the enzymes extracted from the micro-organisms are used. But such an enzyme mixture generally contains various harmful substances and deleterious enzymes. The time of treatment and the concentration of the enzymes to be used depend upon the nature of the culture tissue.
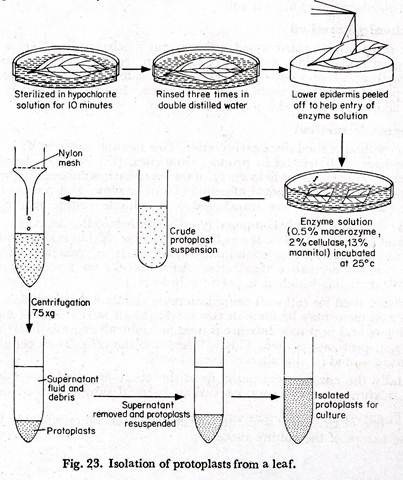
Isolation of protoplasts from the leaves:
(1) Mature leaves of any suitable plant are taken and rinsed in tap water.
(2) The leaves are immersed’ in hypochlorite solution for about ten minutes.
(3) These are washed three times in double distilled water. A culture medium which is adjusted to the pH and osmolarity of the enzyme solution may be used for rinsing the leaves.
(4) With a fine force the lower epidermis is peeled off to help the entry of the enzyme solution (Fig. 23).
(5) The leaves are cut into small pieces.
(6) The leaf pieces are placed in a petridish containing the enzyme solution, having a composition of 0.5% macerozyme, 2% cellulase, 13% mannitol at pH 5.4.
(7) Petridishes are sealed with parafilm and “wrapped with aluminium foils.
(8) These are incubated at 25°C for 12-18 hours. The mesophyll remains in the enzyme solution.
(9) By gentle agitation the protoplasts are released.
(10) The protoplast enzyme mixture is filtered through a nylon mesh. Filtration removes cell wall debris, cell clumps and undigested tissues.
(11) The filtrate is centrifuged. The supernatant fluid contains the debris and is removed by a pipette. The protoplasts remain at the base of the tube.
(12) Isolated protoplasts are re-suspended in the culture medium and the process is repeated thrice.
The protoplasts are ready for culture.
Isolation of protoplasts from a leaf:
Nagata and Takebe (’70) isolated protoplasts of the tobacco leaf. The method used by them is described here. The surface of a tobacco leaf is sterilised with 70% alcohol or 2% sodium hypochlorite solution.
The epidermis is stripped and placed with its strippid surface facing downwards on a medium containing 10% mannitol for three hours. Leaves are cut into small segments. Segments are then placed on enzyme solution containing 0.5% macerozyme, 0.8% mannitol and 0.3% potassium dextran sulphate. It is incubated at 25°G for 2 hours.
The medium is changed at an interval of every half an hour. Isolated cells are washed in 0.8 M mannitol. Cells are transferred to a medium containing 2% cellulose and 0.8 M mannitol for two hours at 36°C. Cell walls are destroyed. Protoplasts are filtered through wire gauge to separate them from the debris and are then collected by centrifugation. Isolated protoplasts are washed in 0.8 M mannitol.
Isolated protoplasts are fragile. So they are suspended in a medium containing sorbitol or mannitol of proper concentration, where protoplasts remain viable. On a medium of proper concentration isolated protoplasts appear rounded.
Isolation of protoplasts from pollen grains:
Bhojowani and Cocking (’72) isolated protoplasts from pollen grain tetrads of several angiospermic plants by using 1 % helicase. The stage of the tetrad is important for protoplast isolation. Early stage of tetrad formation when cell walls are very thin and made up of cellulose is suitable for protoplast isolation.
Ito (’73) isolated protoplasts from pollen grains of plants belonging to Liliaceae by using cellulase and microzyme. Protoplast isolation from myocyte or tetrad in proper stage requires half an hour. In body cell the time required is 4-12 hours.
Protoplast culture:
Protoplast just after isolation is more or less spherical. Protoplasts can be cultured in a micro chamber or in a soft agar medium or in hanging drop culture. Murashige and Skoog’s medium supplemented with auxin and cytokinin helps the growth of the protoplasts.
Addition of 13% mannitol to the medium prevents osmotic lysis of isolated protoplasts. 1.5% agar may be added to the medium. Culture vials are incubated at 25°C in presence of dim white light. For wall formation in Phaseolus calcium chloride and ammonium nitrate are added to the medium.
Within 5-7 days cell wall is formed. The protoplasts divide forming cell aggregates and ultimately a callus. This callus when sub-cultured on a medium lacking auxin and mannitol form embryoids, which produce plantlets.
Takebe and co-workers (’71) obtained entire plant from hybrid protoplasts of tobacco. Nagata and Takebe (’72) cultured isolated protoplasts of tobacco on nutrient medium and obtained multicellular colony. It was then transferred to a fresh medium, where callus was formed. This callus produced buds and shoots.
Significance:
(1) From isolated protoplasts hybrid plants can be produced by protoplast fusion. Even those hybrids, which cannot be produced by normal procedure due to sexual or physical incompatibilities, can be produced by somatic cell fusion.
(2) Isolated protoplasts are required for protoplast fusion experiments forming homokaryons and heterokaryons.
(3) In culture isolated protoplasts soon regenerate new walls. From this, the process of wall formation can be studied.
(4) From isolated protoplasts mutant cells can be selected and clonal propagation can be made.
(5) Isolated protoplasts can inject foreign materials into the cytoplasm by a process resembling endocytosis. The injected material may be a plasmid, bacterium, virus, DNA or mitochondria etc.
(6) It provides materials for plant cell modification experiments.
(7) It helps to solve many plant and cell physiological problems.