In this article we will discuss about the Anther and Pollen Culture:- 1. Nutrient Medium of Anther and Pollen Culture 2. Factors Affecting Anther and Pollen Culture 3. Methods 4. Nurse Culture Technique 5. Significance.
Contents:
- Nutrient Medium of Anther and Pollen Culture
- Factors Affecting Anther and Pollen Culture
- Methods of Anther and Pollen Culture
- Nurse Culture Technique for Anther and Pollen Culture
- Significance of Anther and Pollen Culture
1. Nutrient Medium of Anther and Pollen Culture:
Anthers or pollens can be cultured on a suitable medium containing sucrose (usually 2%), iron, vitamins, hormones etc. The hormonal component of the medium is important for initiation of growth. Usually to the culture medium auxin, cytokinin etc. are added either singly or in various combinations.
Low concentration of auxin stimulates callus formation. In a medium supplemented with auxin embryoid formation usually occurs at a faster rate as observed in anther culture of Datura.
Anthers cultured on a medium containing coconut milk or kinetin develop embryoids which later form haploid plantlets. Callus is formed from pollen grains on a medium supplemented with yeast extract or casein hydrolysate.
In raising haploid plants from isolated pollen culture of Nicotiana tabacum and Datura Nitsch (’74) used a synthetic medium containing glutamine, 1-serine, inositol. In N. tabacum plantlet formation from pollen also depends on iron concentration. In culture of Brassica pollens on Nitsch’s medium (’51) increased sucrose concentration is needed.
2. Factors Affecting Anther and Pollen Culture:
(1) Activated charcoal:
It has a stimulatory effect on embryogenesis and this has been observed in anther cultures of potato, rye, tobacco, etc. This may be due to removal of inhibitory substances from agar by activated charcoal.
Charcoal may absorb the degradation product (5-2-furfural) of sucrose. Anther cultures of Petunia and Nicotiana indicate that activated charcoal removes both exogenous and endogenous growth hormones from culture medium.
(2) Temperature:
Temperature has significant effect on pollen embryoid development. In Datura embryoids are not formed if cultures are maintained at 20°C or below. In Nicotiana tabacum optimal temperature for embryoid growth is 25°C.
Pre-treatment of anthers at 3—10°C for 2—30 days stimulates embryogenesis. Wenzel (’77) observed that buds of Secale cerale pretreated at 6°C for 6—10 days develop embryoids. In N. tabacum if the buds are pre- treated at 5°C for 72 hours than 58% anthers produce embryoids.
Sometimes pre-treatment at high temperature helps embryoid formation. In Brassica campestris pre-treatment of anthers at 35°C for 24 hours helps embryoid formation. Centrifugation of the anthers at 3—5°C for approximately 30 minutes helps embryoid formation.
(3) Stage of the anther:
Particular stage of the anther at the time of culture is important. Usually anthers just before or immediately after pollen mitosis are most suitable for culture. Suitable stages of anthers for culture are pre-mitotic, mitotic and post-mitotic.
(a) Pre-mitotic stage:
Anthers at this stage have microspores which have just completed the first meiotic division and the pollens are immature, uninucleate and starch-free. Anthers of Hordeum vulgare and Hyocyamus at this stage are suitable for culture. According to Nitsch (’72) and Sunderland (’71) anthers with uninucleate pollens are suitable for culture.
(b) Mitotic stage:
In some plants, anthers at first pollen division stage are most suitable for culture, as observed in Nicotiana tabacum and Datura innoxia.
(c) Post-mitotic stage:
Early bi-cellular stage of pollen development is most suitable for culture in Atropa belladonna and Nicotiana sp. etc.
Mature anthers are usually unsuitable for culture, but in Brassica oleracea mature anthers are the proper stage for anther culture.
Anthers of proper stage are chosen by selecting flower buds of definite length under fixed environmental conditions.
(4) Photoperiod and light intensity:
Higher number of embryoids are formed when anthers are taken from plant grown under short days and high light intensities.
(5) Flowering time:
Anthers taken from flowers at the beginning of the flowering period of the plant are most suitable for culture.
(6) Endogenous auxin:
Embryogenic pollens are found near the tapetum within the anthers. The tapetum may release some substance which initiates embryogenic development in pollens. This is observed in Hyoscyamus niger by Raghavan (’78).
(7) Age of the plant:
Usually anthers from younger plants are more suitable for culture.
3. Methods of Anther and Pollen Culture:
Anther Culture:
(1) Selected plants are cultivated until they reach flower bud stage.
(2) In some cases flower buds are chilled few days prior to culture.
(3) Flower buds of proper size and developmental stage are taken and surfaces sterilised with alcohol or hypochlorite solution for 10—20 minutes. Buds are rinsed several times in sterile double distilled water.
(4) The anthers are carefully excised from flower buds using force and dissecting needle. Filaments must be removed prior to culture; otherwise callus may be formed at the cut ends.
(5) Anthers may be cultured either on agar-solidified culture medium or placed on a filter paper bridge over a liquid medium.
(6) Anthers are cultured at 25°G in presence or absence of light. Light is essential after plantlets are formed. Continuous illumination from cool white fluorescent lamp of 300 lux is satisfactory.
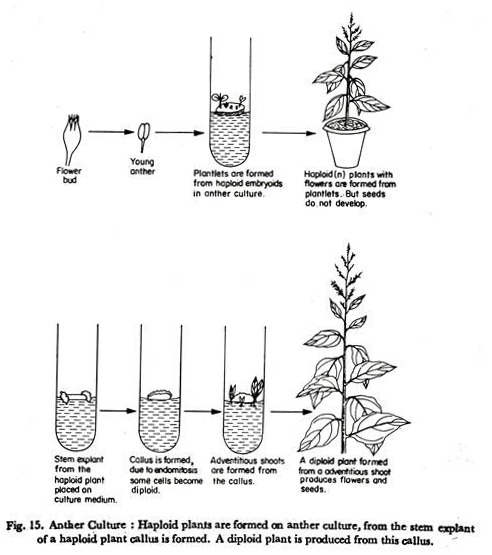
(7) After a period of 4—5 weeks in culture plantlets are formed. From a single anther many plantlets are formed (Fig. 15).
(8) These plantlets are carefully separated quite early and cultured on a fresh root-inducing medium containing 0.5% agar and all other components in half-strength to that of the anther culture medium.
(9) After formation of proper root system they are transplanted to pots. These pots are preferably kept in humid condition for few days.
Nitsch (’72) cultured anthers of tobacco and obtained in 75% of the cultured anthers 1—100 plantlets per anther. Depending on the species and composition of the culture medium the plantlets may be formed directly from the pollens or a callus is formed, from which later plantlets are formed (Fig. 15). Plants arising from an anther are heterogenous.
Pollen Culture:
Stages 1-4 are same as that of anther culture technique:
(5) Anthers are placed in a 5 c.c. liquid medium in a petridish. Pollen grains are removed from the anthers either mechanically or by natural dehiscence. For removing pollen grains mechanically from the anthers a slight incision is made on the anther tissue with a scalpel and its contents are gently squeezed into the medium.
To obtain pollen grains from the anthers Sunderland and Roberts developed ‘float culture’ technique. In this method whole anthers are taken from cold treated buds and are allowed to float on the surface of the liquid medium. Within few days the anthers dehisce and liberate pollen grains at various developmental stages.
(6) Petridishes containing the pollen grains in the culture media are sealed with para-film and incubated at 28°C in dark for fourteen days.
(7) After 14 days the culture is kept in an illuminated chamber at 25°G and a day length of 12 hours.
(8) 3-8 weeks may be required to obtain haploid plantlets.
4. Nurse Culture Technique for Anther and Pollen Culture:
Sharp (’73) working with tomato (Lycopersicon esculentum) developed nurse culture technique for the formation of haploid clones from isolated pollen grains (Fig. 16).
This method in brief is as follows:
(1) An anther is placed horizontally on a semisolid nutrient medium. A filter paper disc is placed over it.
(2) From another anther pollen grain suspension is prepared in a liquid medium having about 10 pollens per 0.5 c.c. of the medium.
(3) Over each filter paper disc 10 pollen grains are transferred. This is incubated at 25 °C in presence of light.
(4) Within a month several haploid clones are formed.
Production of Plantlets from Pollen Grains:
Early pathway of pollen grain development differs in different plants. Uni-nucleate pollen grains divide in culture either equally or unequally into two cells (Fig. 17)’.
(a) If two equal cells are formed by division of pollen grain, then both cells participate in sporocyte formation as in Datura innoxia.
(b) Pollen grains divide unequally forming a small reproductive cell and a large vegetative cell. Sporocyte is formed from the vegetative cell, e.g. Nicotiana tabacum.
(c) Small reproductive cell formed by unequal division of the pollen grain gives rise to the sporophyte, e.g. Hyocyamus niger.
(d) Pollen grain divides unequally and both cells participate in sporophyte formation.
Irrespective of the early pathway of division followed, the pollen grain gradually becomes multicellular. In Atropa, Datura and Nicotiana these cells gradually form a globular embryo which follows normal development.
In this method one haploid plant is formed from one pollen grain. But in most cases the wall of the pollen grain ursts and the multicellular tissue is liberated, which by further divisions form callus. Many embryoids are formed from such a callus (Fig. 18).
Ploidy Level of Pollen Plants:
Embryoids formed directly from the pollen grains are haploid. But the sporophytic plants formed from the callus are mixoploid, because endopolyploidy usually occurs during callus culture. Plants derived from callus are usually diploids and triploids.
Triploids are found in Datura, Petunia, Solanum, Oryza etc. In Oryza sativa haploid to pentaploid plants are produced. Diploid and tetraploid plants may be produced by endomitosis. Triploid and pentaploid plants may be formed on account of nuclear fusion.
Production of Homozygous Diploids:
By doubling the chromosome of the haploid plants produced by pollen or anther culture completely homozygous diploid fertile plants are produced. Chromosome number of haploids can be doubled by various methods.
(1) In chemically induced doubling of the haploid plantlets, the plantlets are treated with colchicine. In Nicotiana tabacum plantlets are immersed in 0.4% solution of colchicine upto 96 hours.
Plantlets are transferred to culture medium after treatment. In mature plants 0.4% colchicine in lanoline paste is used on the upper axillary buds. Terminal bud is removed. This encourages the growth of the lateral buds.
(2) In regeneration by tissue culture diploids may be produced due to endomitosis in callus.
5. Significance of Anther and Pollen Culture:
(1) Pollen culture has great importance in mutagenic studies.
(2) By anther and, pollen culture many haploid plants can be produced very rapidly.
(3) Homozygous diploid plants obtained by doubling the chromosomes of haploids have great importance in plant breeding and crop improvement.