Transplantation of Cell Nuclei:
In 1892, August Weissman proposed that cellular differentiation and specialization might be the consequence of the progressive loss of certain genes from a cell’s genome during successive mitotic divisions.
This hypothesis retained widespread acceptance for more than 50 years until a series of experiments carried out with amphibian egg cells indicated otherwise.
The egg cells of amphibians (e.g., frogs and toads) are large enough to be manipulated using microsurgery.
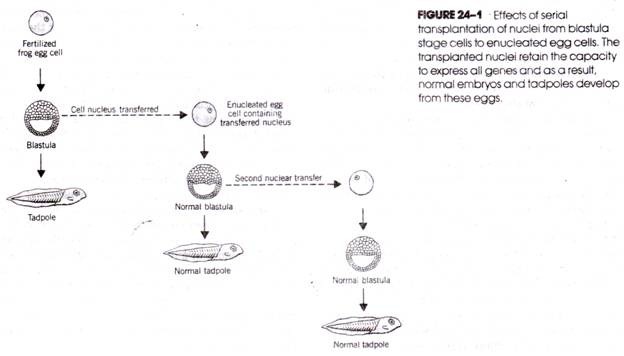
In the 1950s, R. W. Briggs and T. H. King enucleated unfertilized egg cells of Rana pipiens and replaced the haploid nucleus with a diploid nucleus taken from a cell at the blastula stage of development that would have developed into specific embryonic tissues. The cells produced by the nuclear transfer developed into normal frog embryos and normal tadpoles. This demonstrates that determination had not yet taken place in the nucleus of the blastula stage cell. Moreover, the cell still contains all the genes necessary to produce the different types of cells of the complete embryo.
The totipotency of the blastula cell nucleus can be perpetuated even over a series of experimental transfers (Fig. 24- 1), that is, if a nucleus is withdrawn from a blastula stage cell following the first transfer and transferred to a second anucleate egg, the embryonic development of that egg proceeds normally; if a nucleus is removed from a cell at this blastula stage and transferred to a third anucleate egg, normal embryonic development again occurs, and so on.
Whereas the observations of Briggs and King demonstrated that nuclear totipotency exists in cells at the blastula stage of embryonic growth, a series of experiments carried out a decade later by J. B. Gurdon showed that totipotency is retained through much later stages of development. Gurdon removed nuclei from intestinal cells of Xenopus laevis tadpoles and implanted them in enucleated unfertilized egg cells. Some of the resulting cells developed into normal tadpoles and normal, fertile adults (Fig. 24-2).
Thus, even the nuclei of differentiated intestinal cells contain the full complement of genes essential to the production of a complete organism. The experiments with amphibian cells indicate that there is neither a loss of genes during the many mitoses that lead from the undifferentiated fertilized egg cell to the highly differentiated cells of later developmental stages nor an irreversible loss of the capacity to express these genes.
Experiments with Acetabularia:
Classic experiments with the unicellular alga Acetabularia demonstrated that the cytoplasm has a direct effect on gene expression. During the life cycle of Acetabularia (Fig. 24-3), newly formed zygotes attach to the sea bottom by developing root like structures called rhizoids. A slender stalk several centimeters long grows upward from the rhizoid and develops an umbrella like cap.
During growth of the cell, which usually takes several weeks, the nucleus remains in the rhizoid. When growth is complete, the nucleus divides mitotically many times and the resulting daughter nuclei migrate up the stalk into the cap; there meiosis produces haploid nuclei that become encysted. Eventually, the cysts are released, break open, and the gametes swim out. Fusion of two gametes to form a new zygote initiates a new cycle.
Acetabularia is capable of regenerating lost parts. For example, if the cap is cut off, the cell grows a new cap. If the new cap is removed, another cap is grown, and this cycle can be repeated many times with the same individual. If the cap is cut off and the cell nucleus then removed by microsurgery, a new cap is regenerated, but regeneration will not occur if the experiment is repeated with the same anucleate organism.
Regeneration of the second cap in the absence of a nucleus indicates that nuclear information had previously been transferred to the cytoplasm and persisted there, at least through the time required to form the new cap. The information in the cytoplasm for regenerating the cap takes the form of messenger RNA. Different-shaped caps are formed by different species of Acetabularia. Acetabularia mediterranea has a complete cap and A. crenulata has a fingerlike cap. The interactions of the cytoplasm and nucleus are strikingly illustrated by grafting experiments using these two species (Fig. 24-4).
If the foot of A. crenulata (which contains the nucleus) is joined to an anucleate piece of the stalk of A. Mediterranean, the two fuse and a cap develops at the top of the stalk. The cap assumes characteristics of both species, presumably resulting from the combined influences of the cytoplasmic mRNA that was present in the A. Mediterranean stalk and the nucleus and cytoplasmic mRNA that were in the A. crenulata foot.
If the cap produced by the grafted cell parts is removed and a second cap allowed to form the new cap has the A. crenulata shape. Thus, the cap information (mRNA) in the original cytoplasm of the stalk from A. Mediterranean was “lost” after one regeneration, whereas the cap information present in the A. crenulata nucleus is expressed.