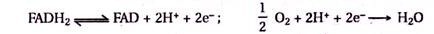Read this article to learn about B Lymphocytes and the Immune Response !
To understand how B lymphocytes are caused to secrete antibodies during an immune response, let’s consider a case in which a person acquires either a bacterial or viral infection.
Two events must generally occur if B lymphocytes are to be activated (Fig. 25- 12).
First, antigens present on the surface of (or released by) the pathogen become bound to antibodies in the plasma membranes of one or more of the millions of clones of B lymphocytes. Binding of the antigen to the surface of the B lymphocytes does not by itself cause activation of the clone. Instead, antigens must also be taken up during nonspecific phagocytosis of antigen-bearing particles by macrophages (i.e., phagocytic cells that act as scavengers in the body’s tissues). The antigens taken up by the macrophages are degraded or “processed” and fragments containing antigenic determinants are then displayed at the cell surface.
Macrophages that carry out this process are referred to as antigen-presenting cells. The antigenic determinant is then recognized by one or more clones of T cells possessing T-cell receptors for the antigen. T cells that recognize and are activated by antigen-presenting cells are called T helper cells.
Activated T helper cells then interact with the B lymphocytes to which antigen had already been bound. The interaction between T helper cells and B lymphocytes serves to activate the B lymphocytes causing the rapid proliferation of the clone, thereby yielding plasma cells and memory cells (Fig. 25-12). Only the plasma cells produce and secrete antibodies. The memory cells are kept in reserve and will be called on to respond during a second (or subsequent) infection by the same antigen-bearing pathogen.
Antibodies secreted by plasma cells may have several different effects:
(1) They may interact with free (i.e., soluble) antigens causing precipitation;
(2) They may interact with surface antigens of the pathogen (i.e., particulate antigens) causing agglutination; or
(3) They may promote complement fixation.
Precipitation of Soluble Antigens:
Antigens may have one or more antigenic determinants (Fig. 25-13). If one antigenic determinant is present, the antigen is said to be mono-determinant; if two are present, the antigen is bi-determinant, and so on. Most antibodies are bivalent, meaning that they can simultaneously combine with up to two antigenic determinants.
As Figure 25-13 illustrates the products formed by interaction of immunoglobulin and antigen depend on the number of antigenic determinants that are present. Two mono-determinant antigens can be cross-linked by a single antibody (Fig. 25- 13a), but the product is not usually insoluble unless the antigen itself is very large. However, if two antigenic determinants are present, cross-linking by the antibody can produce chains of antigens that are insoluble and form precipitates (Fig. 25-13b). Multi- determinant antigens react with antibody to produce cross-linked networks or lattices that are insoluble (Fig. 25-13c).
Interactions between antibodies and free antigens can be considerably more complex than those illustrated in Figure 25-13. For example, some antibodies may exist as dimers (e.g., IgA) or pentamers (e.g., IgM) (see Fig. 25-3); these antibodies can simultaneously bind four or more antigenic determinants. Moreover, antigens may possess more than one kind of antigenic determinant, each determinant capable of reacting with a different antibody.
Finally, the predominant form of interaction that takes place between antibodies and antigens is influenced by the respective concentrations of the interacting species. Small soluble complexes are favored when there is an excess of antibody; chains of cross-linked antigens are favored when there is an antigen excess; and cross- linked lattices are favored by nearly equal amounts of antibody and antigen. Regardless of the nature of the products formed, antigen-antibody complexes are eventually eliminated by the phagocytic action of macrophages.
Agglutination:
Antibodies that interact with antigens present in the surfaces of invading microorganisms or other foreign particles cause agglutination (Fig. 25-14). During agglutination the particles become cross-linked to form small masses, and the masses are eliminated by the phagocytic action of macrophages.
As illustrated in Figure 25-14, the plasma membranes of macrophages possess receptors that recognize and bind the C-terminal or Fc regions of immunoglobulin heavy chains (see Fig. 4-35). Consequently, the macrophage receptors are called Fc receptors. Because the Fc regions of the immunoglobulin’s include constant domains, macrophage Fc receptors can bind a variety of different antibodies. Interaction between a macrophage and a mass of agglutinated cells is followed by phagocytosis.
Although the mechanism is not fully understood, foreign cells that have attached antibodies can also be destroyed by K (or killer) cells. Killer cells bind the agglutinated mass by interacting with the Fc regions of antibodies but do not internalize it. Instead, it is thought that there is the transfer of toxic substances from the K cell to the pathogen.
Complement Fixation:
The complement system is part of still another mechanism by which antibodies defend the body against invasion by pathogens. Complement consists of more than a dozen proteins that circulate in the blood. The binding of antibodies to a cluster of antigenic determinants in the surfaces of bacteria triggers a cascade of reactions in which the complement proteins (many of which are proenzymes) are sequentially activated.
The cascade is initiated by the binding of a small complex of the complement proteins to the constant regions of antibodies that are bound to the bacterial antigens. In the ensuing reactions, additional complement proteins are bound and activated, eventually forming a lytic complex that creates an open channel through the bacterial surface.
By disorganizing the bacterium’s plasma membrane and allowing water to enter the cell by osmosis, the bacterium is killed. Complement fixation by antibody-coated bacteria and the lysis of the invading cells that follows is the most common defense mechanism attributable to B-cell- secreted antibodies.
Immunologic Memory:
Figure 25-15 shows the relationship between time and the appearance of antibodies in response to a first exposure to a given antigen. Following a short lag period, antibodies begin to appear in the blood, rising to and maintaining a plateau level for some time before falling again. This characteristic response curve is called a primary immune response.
As long as the antibody content of the blood remains at its plateau level, a condition of active immunity exists. The response to a second exposure to the same antigen—the secondary immune response—is much more dramatic.
The lag period is shorter, the response is more intense (i.e., greater quantities of antibody are produced) and the elevated antibody level is maintained for a longer period of time. The difference between the two responses indicates that the body has “remembered” its earlier exposure to the antigen.
Immunologic memory may be explained in the following way. The initial exposure to antigen causes differentiation of B lymphocytes into memory cells as well as plasma cells. Whereas the plasma cells have a relatively short life span in which they are actively engaged in antibody secretion, memory cells do not secrete antibody and continue to circulate in the blood and lymph for months or years. These memory cells are able to respond more quickly to the reappearance of the same antigen than undifferentiated B lymphocytes. Memory cells are also produced by the multiplication and differentiation of T lymphocytes.
Autoimmune Diseases:
The immune system normally produces antibodies against foreign proteins but not against the native proteins of the body, that is, the immune system can distinguish between “self” and “non-self.” Yet one’s own proteins will readily be regarded as antigens by the immune system of another organism. Thus, each individual’s tissues possess a myriad of proteins (and other chemical substances) that are potential antigens.
The capability to distinguish self from non-self develops very early in life. In the 1950s, P. B. Medawar carried out a series of elegant experiments that bear on this concept. Adult mice from one strain reject skin grafts from another strain; that is, the recipient’s immune system produces antibodies against antigens in the donor’s tissue and this leads to the destruction of the donor’s cells.
However, when living spleen cells (which carry the same antigens as skin cells) from one strain of mice were injected into newborn mice of a different strain and the skin graft experiments repeated when the newborn mice reached adulthood, the results were entirely different.
Newborn mice that had been exposed to the spleen cells of another strain accepted skin grafts from that strain later in life. This is interpreted to mean that the spleen cells had been transferred to the new born mice while the mice were at an early enough stage of development to accept the spleen cells as “self’ by the maturing mouse immune system.
In rare cases, individuals begin to produce antibodies against their own antigens. These antibodies are called autoantibodies and the diseases resulting from their presence are the autoimmune diseases. Among these diseases are paroxysmal cold hemoglobinuria (antibodies against one’s own red blood cells), myasthenia gravis (antibodies against one’s own muscle cell acetylcholine receptors), and systemic lupus erythematosus (antibodies against one’s own nuclear DNA).
The causes of autoimmune diseases are not entirely clear and several different mechanisms seem to be involved. Clones of lymphocytes prepared to respond to a non-self (i.e., foreign) antigen that is structurally similar to self may undergo mutation during clonal expansion, thereby producing cells that now respond to self.
It has recently become clear that T and B cells re active to self antigens are present even in normal individuals. However, in normal individuals T suppressor cells serve to suppress the activity of these cells and thereby prevent autoimmune diseases.