In this article we will discuss about:- 1. Basic Strategies for Secondary Metabolite Production 2. Factors Affecting the Production of Secondary Metabolite Production 3. Specialized Strategies 4. Regulation.
In the beginning of the plant tissue culture, studies were heavily focused on the fundamental assessment of nutrition, growth and differentiation. Several advancements in cell and tissue culture techniques have taken place after 1960 and have paved new avenues for commercialization of in vitro techniques.
Realities on potentialities of plant cells for the in vitro production of secondary metabolites gained a significant momentum during this period. The contribution of Jones and his associates on the cell culture techniques laid an initial foundation for the production of secondary metabolites.
Plants are armed with several fine chemicals which are of immense value in pharmaceutical industry. There have been several persistent endeavours to accomplish tissue culture strategies for the production of pharmaceutically important secondary metabolites. The most commonly referred plant secondary metabolites are phytochemicals, which includes alkaloids, plant phenolics and massive diverse group of terpenes.
Plants are armed with these phytochemicals which are expressed basically in response to environmental factors such as ultraviolet, light intensity and defence related functions against microbial and pest attacks. Earlier conclusions on secondary metabolites have shown that their production in plants do not participate in growth and development of plants.
But, recent evidences proved that these phytochemicals are actively participating in plant defence functions and signal mechanisms. Primary metabolites contribute overwhelmingly in the production of secondary metabolites by specialized pathways common to all plants.
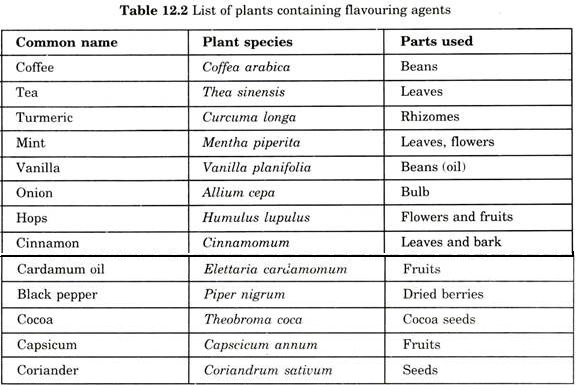
Several secondary metabolites are useful in cosmetic industries which include shikonin and aloe juice for skin treatment. In addition, several insect repellants, colouring and flavouring agents have also been noticed as potential useful compounds (Table 12.2).
Realizing the economic potential of high demanding phytochemicals, cell culture technology has become increasingly significant for the production of secondary metabolites. The first commercial production of shikonin by cell culture using Lithospermum erythrorhizone in 1983 has attracted the researchers at a global scenario. The number of patents issued in Japan shows that secondary metabolites clinched major share of patents during 1975-1985.
Although secondary metabolite production in cell culture is feasible, their economic factors for enhanced production of most of the target metabolites are still handicapped. Therefore, refined cell culture strategy is required to address these problems in booming new technology for secondary metabolites.
Some of the far most advantages of metabolite production over conventional methods are:
(a) In vitro culture, which ensures production of metabolites throughout the year irrespective of the season. On the contrary, production and accumulation of phytochemicals takes place only during specific season in field grown plants.
(b) In vitro production of phytochemicals takes place within a short span of time when compared to the natural plants.
(c) In vitro culture could also ensure high level production of secondary metabolites.
Basic Strategies for Secondary Metabolite Production:
Callus:
Callus, a homogenous mass of undifferentiated cells can be obtained from all parts of the plant body. In culture, callus can be obtained by placing explants onto a growth supporting media. Generally, callus-inducing media is supplemented with either auxin alone or in combination with low or equal concentration of cytokinin. Auxins such as 2, 4-D, IAA, NAA can yield substantially better results.
The process of cell differentiation occurring in the plant is reversed and the explant tissue becomes dedifferentiated. In vitro raised callus generally exhibits two types—Nodal and Friable types. Friable nature of callus contains loosely aggregated cells which are useful for the production of secondary metabolites. Friable nature can easily transform callus into suspension culture.
Friability of the callus is due to neutralization of galactoside carboxyl group, which reduces the possibility of cross linkage between polysaccharide chain and unequal esterification of the carboxyl groups. It is possible to enhance friability by creating folic acid deficiency in culture medium and increasing vitamin B12 concentration. Callus system has been exploited for the production of secondary metabolites.
Callus culture of saffron and capsaicin, established on Murashige and Skoog medium produced high level of these metabolites. Similarly, the possibility of steroidal sapogenins production upto 2% was noticed in undifferentiated cultures of Diascoria deltoidea. But differentiation of organs results in the decrease of the sapogenins in culture. Insecticidal compound pyrathrine was produced successfully in the callus culture of Chrysanthemum cinerarifolium.
Biosynthetic ability of flavour imparting compound thymol in the callus culture of Carum copticum was found to be greatest in the semi-organized cultures than in unorganized cultures. On several occasions however, alkaloid formation takes place in the shoots regenerated directly from the plant without the callus.
Initiation of Suspension Culture from Callus:
Suspension culture is the mass of free cells or cell clumps in liquid medium. It is generally obtained by transferring the friable callus into agitated media of the same composition as that used for callus growth. Initially, callus is cut into small pieces and transferred into the medium. Generally, large callus inoculum is advantageous as it ensures sufficient release of free cells or cell clumps into the liquid medium. Presence of cell clumps and high cell density facilitates cell to cell contact.
Agitation in one form or the other has been used to facilitate fragmentation of the cell groups and helps in diffusion of metabolites and oxygen. The agitation rates on orbital shaker should be in the range of 30-150 rpm with an orbital motion strike of 2-4 cm. However, plant cell culture requires 90-120 rpm for most of the species (Fig. 12.2). In order to procure fine suspension culture, frequent sub-culturing is indispensable into the fresh medium.
Presence of large cell clumps can be removed by pipette or syringe of suitable orifice diameter to exclude large cell aggregates. Some of the callus types such as nodular or compact callus do not readily breakup to form suspension. It is also possible to increase friability of the callus by repeated subculture. On several instances cell suspension culture was employed as a sole strategy for enhanced production of secondary metabolites.
Types of Suspension Cultures:
(i) Batch Culture:
It is a type of suspension culture in which cells are grown in fixed volume of the nutrient medium. In most of the batch cultures, volume of the suspension is maintained between 250 mL and 10 L (Fig. 12.2). Increase in the density of the cell suspension is an indication of increase in biomass. Cell division and growth continues until the nutrient or oxygen controls the cessation of the cell growth.
The growth kinetics of cells in suspensions undergoes four phases of growth cycle, (a) Lag phase — in which cells prepare to divide, (b) Exponential phase — in which rate of cell division attains maximum, (c) Linear phase — in which reduction of cell division takes place, (d) Stationary phase — where the number and the size of the cells remains constant.
A lag phase is the beginning or the initial phase of the growth cycle. This phase is sometimes crucial for the synthesis of certain specific metabolites. This is followed by the exponential phase, where rapid cell division proceeds resulting in increased biomass. After few cell generations (2-3) growth rate is retarded in linear phase. The last and final stage is the stationary phase during which cell dry weight is reduced. Sub-culturing is followed regularly for every 2-3 days in exponential phase (Fig. 12.1).
(ii) Continuous Culture:
The cells in suspension culture can be maintained for a considerable period of time by replacing with fresh media and cell population. There are two types of continuous culture systems — closed continuous and open continuous culture systems. In closed continuous culture system, the spent medium in the culture vessel is removed and outflow is balanced by inflow of fresh medium.
The cells from outflowing medium is retained and pushed back into the culture. The cell biomass increases continuously in this system. In open continuous culture, inflow of fresh medium is balanced by outflow of spent medium of the same corresponding volume. The cells are harvested and replaced by fresh batch of cells.
One of the advantages of this system is that cell cultures are maintained indefinitely at a constant maximum growth rate. The open continuous culture system is again divided into chemostat and turbidostat. In chemostat, growth and cell density are maintained constantly by fixed rate of input of growth limiting elements like nitrogen and phosphorous.
In turbidostat, inflow of fresh medium takes place in response to increase in turbidity, thus facilitating continuous maintenance of culture at a fixed optimum density of the suspension. The growth of cell suspension culture may be monitored by measurement of one or more of the parameters such as packed cell volume (PCV), cell number, wet and dry weight, protein and DNA content and medium conductivity.
Determination of cell density by PCV method involves transfer of a known volume of suspension to 15 mL of graduated centrifuge tubes and spin for 5 min at (200 × g). PCV is the volume of cell pellet and is usually expressed as percentage of the cell numbers in suspension. The cell number in suspension may be counted directly by haemocytometer.
Bergamann Cell-Plating Technique for Single-Cell Culture:
Embryogenic cell suspensions are potential source for the production of several fine chemicals and offers large-scale clonal propagation. Thus, it is desirable to produce single cell clones for useful purposes. The technique of single cell culture was shown by Bergamann in 1960, where cell suspensions are initially plating out on agar plate. The technique involved counting of cells from suspension culture in order to adjust density by dilution of the suspension culture.
Equal volumes of agar medium of appropriate temperature (35°C) and suspension are mixed and dispersed into the petri dish and uniform distribution of cells are ensured. The suspension is filtered through a sieve and fine suspension is plated accordingly. The sealed dishes contain cells which are incubated at 25°C for 21 days. The number of cell colonies formed in dishes is counted by photographic document.
Factors Affecting the Production of Secondary Metabolite Production:
Plant Material:
Genetic sources of plants have been implicated in the productive level of secondary metabolites. It has been proved that certain plants known as high-yielding cultivars can produce high amount of secondary metabolites in culture as shown in Catharanthus roseus.
Similarly, high-yielding tobacco in culture, accumulated increased level of nicotine than cultures derived from low-yielding cultivar. Therefore, it is possible to recover high amount of fine chemicals from high-yielding plants irrespective of explant source.
Media Components:
Although formulations of Murashige and Skoog’s (1962) medium were originally designed for rapid growth of tobacco, it is widely employed even for secondary metabolite production. This medium does not support metabolite production despite its role in rapid growth of the tissue. Secondary metabolite production is generally accomplished by two types of media.
One is growth medium, which encourages rapid growth of the cells leading to increased biomass and, second one is production medium, which induces production of secondary metabolites. Zenk was one of the first persons to use a two-stage culture, in which growth and production medium was combined for the culture of several plants.
In the production of shikonin, a particular MG-5 growth medium from Linsmayer and Skoog (1965; LS medium) was designed and M-5 production medium derived from White’s medium by enhancing the concentration of the nutrients in each medium. A similar success was also noticed in the production of barberine by using two stage cultures.
The composition of the macro-elements of the media can control secondary metabolite production. Several media like Linsmayer and Skoog (1965) and Gamborg (1968), which were originally designed for cell growth, have been modified extensively to suit successful production of secondary compounds. Shikonin was not produced on LS medium, which was originally designed for cell growth.
However, shikonin production was noticed once the cells were transferred onto the White’s medium. Suppression of shikonin production on LS medium is probably due to the presence of high level of NH4, whereas, White’s medium is devoid of NH4, supported shikonin production. It was however shown that removal of NH4 from LS medium could support shikonin production.
In addition to ammonium as the limiting factor, direct reduction of other media components such as nitrate and phosphate results in the production of secondary metabolites. For instance, production of ajmalicine and serpentine occurs when phosphate level is reduced in the medium. Similarly, a spice compound capsaicin is produced when nitrogen level is lowered in the medium.
Cell Density:
Culture vessel or bioreactor containing appropriate level of cell density, stimulates secondary metabolite production. The maximum cell density is accomplished when the tanker filled with cells, i.e., 30—120 g dry weight of cells is being packed in 1 L of the bioreactor. Most of the culture shows maximum cell yield of about 15 g per litre. This cell filling culture can be exploited commercially.
The successful cell filling technique with maximum cell density is possible only when adequate agitation is pressed without cell destruction, sufficient oxygen and continuous supply of nutrients. The cell density upto 70 g dry weight per litre was possible by adopting the above parameters. Increased aeration causes the cells to attach to the upper part of the bioreactor wall. In order to control this, the aeration rate must be manipulated and kept low by mixing oxygen with aeration gas.
Growth Regulators:
Production of secondary metabolites in vitro culture is due to the cell multiplication and division. This process invariably requires growth regulators. Participation of growth regulators in metabolite production is well documented in vivo. There are several instances of in vitro culture in which secondary metabolite is produced under the influence of growth regulators.
In the suspension culture of Papaver bractiatum, efficiency of different auxins was compared for Thebaine production. Auxin like IAA was found to be superior to NAA and 2, 4-D in culture for the production of anthoquinine. NAA was found to be highly beneficial whereas, 2, 4-D has negative effect on its production.
Similar results on the poor performance of 2, 4-D was seen in the callus culture of Nicotiana tobaccum for the production of nicotine and anabasine, which was successful with IAA supplied medium. At this juncture it can be concluded that 2, 4-D is a poor candidate in inducing secondary metabolite production. The possible reason for 2, 4-D’s poor response in culture is due to the lower pools of glutamate and aspartate.
After IAA, another ideal candidate NAA, exhibits good response in the production of nicotine. Barring few instances of involvement of cytokinins in inducing accumulation of steroidal sapogenins, reports are still scanty. Plant hormone like gibberellic acid (GA) facilitates diosgenin production in Diascorea cultures.
Carbon Source:
Efficacy of various carbon sources for secondary metabolite production has been tested. Among all carbon sources tested, sucrose is suited for most of the plants. Positive role of sucrose was noticed on nicotine production by tobacco callus. Glucose on the other hand acts as suitable carbon source for supporting secondary metabolites production.
Light:
In tissue culture, low radiant level of broad spectrum quality not only controls various morphogenesis but also influence secondary metabolite production. Several enzymes, which are involved in the biosynthetic pathways leading to the production of cinnamic acid, coumarins, flavanols, chalcons and anthocyanins are controlled by light. For instance, increase in the activity of enzymes of the flavanol pathway takes place when cells are exposed to light resulting in anthocyanin accumulation.
One of the active spectrum regions in blue light increases the activity of phenyl ammonia lyase (PAL). The blue light induction of PAL and other enzymes after exposing parsley cell culture to cool white fluorescent light favours metabolite accumulation. Induction of polyphenol has been noticed in tea callus culture. In addition, continuous light illumination results in the accumulation of catechin and epicatechin in suspension culture.
Temperature:
Generally, tissue cultures maintained at 25°C favours overall growth. But, several evidences have shown that temperature alone can induce secondary metabolite synthesis. More than 100% nicotine accumulation takes place in tobacco seedling exposed to 21°C or 32°C. Differential effect of various temperatures were obtained in the alkaloid production in Peganum callus culture, in which 30°C favours callus growth whereas 25°C stimulates alkaloid accumulation in culture.
Specialized Strategies for the Production of Secondary Metabolites:
Supply of Precursors:
Although, plant cells are totipotent in carrying out secondary metabolic pathways, plant cells in culture generally shows low-level production of secondary metabolites when compared to the natural plants. Only scanty information is available on the exact factors which control metabolite production.
One of the in vitro constraints is that secondary metabolite pathways are blocked by one or more deficient intermediates. To overcome the lacuna, intermediates are supplied in culture media to set right the pathways. Timing of precursor supply is very important because precursor when fed initially may inhibit both cell growth and metabolite production.
Callus culture of Capsicum annum shows that the flavour component of capsaicin is synthesized from valine and phenyl alanine and also can be manipulated by low level of nitrogen and elimination of sucrose in the medium. Capsaicin production is further enhanced by supplying immediate precursors like vanillylamine and isocarpic acid.
Increased production of diosgenin is accomplished by feeding cholesterol (100 mg/L) to Dioscorea culture. Similarly, feeding of phenylalanine to the culture of Coleus blumei enhanced the production of rosemarinic acid. In all these classic examples, timing of precursor feeding has been found to be significant.
Elicitors:
Elicitors are the triggering factors which can elicite the production of secondary metabolites. These are the substances originally derived from microorganisms and actively participate in secondary metabolism. Elicitors are of two types—abiotic and biotic elicitors. Abiotic elicitors are light and metallic co-factors and others. Certain particular wavelengths of the light (Ultraviolet (UV) has a significant influence in the induction of secondary metabolites, for example, flavon glucoside synthesis is most sensitive to UV light at wavelength below 300 nm.
Similarly, induction of anthocyanine takes place at the peak of 312 nm and 438 nm. Certain metallic co-factors such as gold, copper and silver are added into the medium which can induce secondary metabolite production by acting as abiotic elicitors. These metabolic co-factors can be a part of the enzymatic activity involving secondary metabolic pathways.
Most of the elicitor systems employed in culture systems are biotic elicitors, mainly fungal extractions. Biotic elicitors can elicite the production of phytoalexins in culture as part of a plant-defence mechanism against pathogens. Phytoalexins are a diverse group of compounds like isoflavonoids and phenylpropanoids.
Biotic elicitors are simply the extracts of fungi and fungal cell wall material, which are generally included in the medium. Soyabean cell culture when inoculated with the strain of Pseudomonas syringa, triggered glyeollin (phytoalexin) production. Addition of culture filtrate of micromucor to the cell culture of Catharanthus roseus enhances tryptomine biosynthesis.
Elicitors are also probably involved in controlling gene expression. As a result, increased level of enzyme production can stimulate the synthesis of compounds which are new to the cells. Fungal elicitors when supplemented into tobacco cell suspension result in the induction of sesquiterpenoids, like capsidiol.
Imposition of stress on the cell culture triggers secondary metabolite production. Accumulation of indol alkaloids takes place by supplementing a stress hormone abscisic acid into the suspension culture of Catharanthus roseus.
Designing of Bioreactors:
Maintenance of cell suspension culture for considerable period of time essentially requires certain facilities such as aeration, monitoring of O2 and CO2 level and replacement of spent media. Continuous culture system generally fulfills all the conditions essential for long-term maintenance. It is however indispensable to design a suitable bioreactor convenient for cell growth and production of secondary metabolites.
Some of the commonly used bioreactors available for large scale culture of cells are stirred tank bioreactors. Several types of stirred tank bioreactors including low-speed tank minimize cell damage and degradation during culture. Modified version of stirred tank like air lift bioreactor has been found to be well suited for plant cell growth (Figs. 12.3 and 12.4) depending on air lift loop vessels have several advantages like low shear, proper mixing of cells, no sedimentation and minimal cell lysis.
Fujira and Tabota (1987) designed two culture tanks suitable for the culture of Lithospermum erythrorhizon for the production of shikonin. Although paddle impeller supports cell growth of Lithospermum, the average yield of shikonin was found to be lower than in the regular flask culture.
Increase in rotatory speed of the impeller reduces shikonin production. Rotary drum tank was designed to overcome this constraint and was successful in the culture of Lithospermum. One of the key advantages of rotary drum cultures is low injury to the cells due to its gentle mix by the revolution of the tank. As a result, cells are adhered to the wall of the tank.
Immobilization:
Imprisonment of plant cells for secondary metabolite production has several advantages. This can be used to prolong production phase and thus total product yield has been achieved. This newly designed strategy of cell immobilization can ensure rapid growth of cells in suspension and secondary metabolite production.
Entrapment of cells or tissue can be accomplished by variety of gel entrapment or encapsulation systems in which, different gel matrices like agar, gelatin, agarose, sodium alginate, cellulose and polysaccharides are employed. Several simple and non-toxic materials such as sodium alginate, polysaccharide, agar agar and xanthan gum are used for cell immobilization.
The technique is simple, cheap and gives high surface area to the volume ratio. The cells or cell aggregates can be imprisoned within the inert matrix, permitting easy flow of liquid media across the cell. Capsaicin production by immobilization of cells and placental tissue of Capsicum annum was compared and analyzed that immobilized placental tissue exhibits greater potential for capsaicin synthesis than immobilized cells.
Placental tissue is the site of capsaicin synthesis. Addition of elicitors, curtlan, was effective on capsaicin production in immobilized cells. In addition, several cell cultures of Catharanthus rosues, Morinda citrifolia and Daucas carota have been successfully immobilized by polyurethane and polyester.
Several bioreactors have been designed for accumulation of immobilized cells in gel matrix. Utility of fluidized flatbed system is effective in maintaining entrapped cells. The modified version of flat-bed system is column culture in which vertical reservoir containing nutrient solution and culture vessel enclosed entrapped cells. The nutrient media present in the reservoir is sprinkled on entrapped cells by drop arrangement (Fig. 12.5).
Several advantages are associated with immobilization of cells for the production of secondary metabolites. Immobilization of cells increases cell to cell proximity, communications and cell to cell interactions. This is possible during adhesion of cells after division. Variation of cell shapes in terms of the size and number shows heterogeneity of culture system and science of organ initiation or pro-embryoid occurring in foam-immobilized culture.
Other advantages are the release of products into the medium instead being retained within the cells. Removal of secondary metabolites for analysis is possible without any constraints. Therefore, immobilized cell techniques are preferred over other methods for the basic investigations of product biosynthesis and manipulation without affecting cultural system. In addition, the production of H2 gas as a fuel has been analyzed by immobilization of cyanobacterial cells.
Biotransformation:
Biotransformation is one of the major thrust areas of biotechnological applications of plant cell and tissue culture. It is the production of the valuable products by plant cells in culture from cheap precursors which cannot be transformed by chemical or microbial systems. In addition, it is a novel approach to synthesize the substance of unknown structure and exhibits new pharmaceutical properties in cell culture.
There are two methodological approaches followed in biotransformation. One is to obtain novel compounds from substrates that are not normally available to the plants or products from other species. The second approach is to transform natural intermediates of important plant products.
One of the most classic examples and widely proven potential is the biotransformation of cardenolides, is of special pharmaceutical consideration because of glucosides which are widely used in medicines for heart disease. Digitoxin and digoxin are the two important pharmaceutical compounds extracted from Digitalis lanata.
Therapeutically digoxin is superior to digitoxin. D. lanata, however, contains high level of digitoxin. Digoxin is different from digit oxin only by a hydroxyl group at C12 region. Biotransformation of digitoxin to digoxin takes place by cell culture of Digitalis to several products. Some of which are hydroxylated at C12 and produces digoxin.
The Digitalis lanata cell culture strain 291 cultivated in air lift bioreactor was found to be effective in the conversion of P-methyl digitoxin into p-methyl digoxin. In addition, ability of some cultures of D. lanata to effect glucosylation, hydroxylation and acetylation were established. Several examples of biotransforming cited in the cell culture are listed in Table 12.3.
Immobilized cells are used to bio-transform chemical substrates in the production of useful compounds. Immobilized cells exhibit more stability and inflict biotransformation more efficiently than cells in suspension system.
The efficiency of biotransformation still needs to be investigated due to lack of preliminary knowledge of biosynthetic pathways and its related enzymes involved. However, many bio-transformations using plant cells that have potential industrial applications are hydrogenation, hydroxylation and hydrolysates.
Hairy Root Culture:
The potentials of hairy root cultures from plant transformation by Agrobaderium rhizogens for the biosynthesis of secondary metabolites have been well documented. Hairy roots can be produced by incubating a piece of plant tissue in Agrobacterium rhizogens solution. Transformation takes place due to the transfer of T-DNA from bacteria into the plant cells.
The Ri plasmid of A. rhizogens contains auxin-related genes in T-DNA. The successful integration of T-DNA inside plant DNA resulted in the expression of auxin-related genes and consequently, over production of IAA. As a result, numerous hairy roots are induced from the explant. Several examples on the secondary metabolite production by hairy root culture have been reported.
Production, accumulation and release of nicotine and nicotine-related alkaloids are facilitated by hairy root culture of Nicotiana rustica and the accumulation of betacyanine and betaxanthin in Beta vulgaris. There have been reports on the production and secretion of novel therapeutic protein using hairy root culture. Hairy root cultures are novel possible potential sides for the synthesis and easy secretion of recombinant proteins. Thus, avoiding expensive downstream processing.
Altered DNA Methylation:
Role of DNA methylation in the regulation of gene expression has been established. Hypermethylation decides the nature of expression of genes. Hypomethylations are believed to be involved in hypergene expression. Synthesis of secondary metabolites involves participation of several enzymes in these pathways.
Any interference in their production may hinder the synthesis of metabolites. There have been reports on the enhanced production of secondary metabolite by hypomethylation process. Azacytidine is used as an effective chemical in cell culture for the induction of DNA hypomethylation.
Regulation of Secondary Metabolic Pathways:
The current knowledge about the regulation of metabolic products in plant cell culture is scanty and available only in few examples like:
(a) Influence of media on secondary product,
(b) Role of key enzyme for the regulation of secondary product,
(c) Degradation of secondary product and
(d) Inhibition of synthesis.
Concept on two-step culture has been implicated in microbial system. In plant cell culture proposal on two-step culture media was found to be suited for secondary metabolite production. As described earlier, first media is meant for cell growth and the second media for secondary metabolite induction.
The real breakthrough in the metabolite production is achieved when nitrate or phosphate or both are reduced in the media. Downsizing of sugar concentration can have greater influence on secondary metabolite production. Phytochemical production in Catharanthus rosues and Nicotiana tobaccum was achieved by employing induction medium, in which both phytohormones and inorganic phosphates are totally removed from the medium.
It was conceded that copper in the medium has no influence on growth. But, its concentration several times stronger than in White’s medium can induce shikonin production at increased rate. The dependency of the secondary metabolite production in the requirement of phytohormones has not been characterized. In some instances, high doses of growth factors can increase the production of secondary metabolite.
The role of certain enzymes in the regulation of secondary compound production cannot be ruled out. The production is correlated with the activity to enzyme that links primary and secondary metabolic pathways. Gene-cloning technique has laid a foundation in understanding regulatory activity of enzymes. One of the best studied examples of regulation of secondary metabolism in plant cell culture is the induction of enzymes catalyzing the synthesis of isoflavanoids and flavanoids.
In the cell culture of Glycine max flavanoids are induced by spontaneous process or light. Similarly irradiation by ultraviolet can decide accumulation of flavons. In addition, certain enzymes like phenylalanine ammonialyase (PAL) activity to be a crucial factor in the regulaton of secondary compound in several plants.
Degradation of secondary compound is a major factor determining alkaloid content of the plant. It was able to show that quinolizidine alkaloid in the cells and medium of Lupins cell cultures are subjected for fast turnover and probably regulated by light. Regulation of secondary compound sterol production was revealed by the use of sterol inhibitor when a particular enzyme in the pathway is blocked.
Some plant bioregulatros exhibit their effect on plant growth by interfering with gibberellic acid biosynthesis. For example, 2-chloroethyl, 1-trimethylammonium chloride known to inhibit the cyclization of geranyl-geranylpyrophosphate. They also show activity on triterpenoid metabolism and carotenoid metabolism.