Read this article to learn about the applications of secondary metabolites and the production process of secondary metabolites in plant cultures.
The production process comprises of seven aspects.
The seven aspects are: (1) Selection of cell lines for high yield of secondary metabolites (2) Large scale cultivation of plant cells (3) Medium composition and effect of nutrients (4) Elicitor-induced production of secondary metabolites (5) Effect of environmental factors (6) Biotransformation using plant cell cultures and (7) Secondary metabolite release and analysis.
Contents
Secondary Metabolites:
The chemical compounds produced by plants are collectively referred to as phytochemicals. Biotechnologists have special interest in plant tissue culture for the large scale production of commercially important compounds. These include pharmaceuticals, flavours, fragrances, cosmetics, food additives, feed stocks and antimicrobials.
Most of these products are secondary metabolites— chemical compounds that do not participate in metabolism of plants. Thus, secondary metabolites are not directly needed by plants as they do not perform any physiological function (as is the case with primary metabolites such as amino acids, nucleic acids etc.). Although the native plants are capable of producing the secondary metabolites of commercial interest, tissue culture systems are preferred.
The advantages and limitations are listed:
Major Advantages:
1. Compounds can be produced under controlled conditions as per market demands.
2. Culture systems are independent of environmental factors, seasonal variations, pest and microbial diseases and geographical constraints.
3. Cell growth can be controlled to facilitate improved product formation.
4. The quality of the product will be consistent as it is produced by a specific cell line.
5. Recovery of the product will be easy.
6. Plant cultures are particularly useful in case of plants which are difficult or expensive to be grown in the fields.
7. Mutant cell lines can be developed for the production of novel compounds of commercial importance, which are not normally found in plants.
8. Biotransformation reactions (converting specific substrates to valuable products) can be carried out with certain cultured cells.
9. The production control is not at the mercy of political interference.
10. The production time is less and labour costs are minimal.
Considering the advantages listed above, about 25-30% of medicines for human use, and the various chemical materials for industrial purposes are obtained from plant tissue cultures. In general, tissue culture production of natural materials is cheaper compared to synthetic production. However, there are certain limitations associated with tissue cultures.
Limitations/Disadvantages:
1. In general, in vitro production of secondary metabolites is lower when compared to intact plants.
2. Many a times, secondary metabolites are formed in differentiated tissues/organs. In such a case, culture cells which are non-differentiated can produce little.
3. Cultured cells are genetically unstable and may undergo mutation. The production of secondary metabolite may be drastically reduced, as the culture ages.
4. Vigorous stirring is necessary to prevent aggregation of cultured cells. This may often damage the cells.
5. Strict aseptic conditions have to be maintained during culture technique: Any infection to the culture adversely affects product formation.
Why do Plants Produce Secondary Metabolites?
Based on the existing evidence, it is believed that the production of some secondary metabolites is linked to the induction of morphological differentiation.
Consider the following examples:
1. Cardiac glycosides are found in the leaves of Digitalis.
2. Quinine and quinidine are present in the bark of Cinchona.
3. Tropane alkaloids (e.g. atropine) are found in the roots of Atropa.
It appears that as the cells undergo morphological differentiation and maturation during plant growth, some of the cells specialise to produce secondary metabolites. It is also observed that in vitro production of secondary metabolites is much higher from differentiated tissues when compared to non- differentiated or less differentiated tissues.
Applications of Secondary Metabolites:
From the time immemorial, man has been dependent on the plant products, besides the supply of food from plants. These plant products, mostly the secondary metabolites include pharmaceuticals, flavours, perfumes, agrochemicals, insecticides and raw materials for industries. Chemically, the plant products may be alkaloids, terpenoids, glycosides (steroids, phenolics) etc.
As and when available, the natural plant products are preferred to synthetic products, by man. According to a WHO survey, nearly 70-80% of the world population depends on herbal drugs. It is a fact that many chemicals with complex structures that cannot be chemically synthesized can be conveniently produced in plants.
The production of speciality chemicals by plants is a multibillion industry. The plant cell cultures provide laboratory managed sources for the supply of useful plant products. Although hundreds of new compounds are identified every year in plants, only a few of them are of commercial importance. Attempts are made to produce them in cell culture systems.
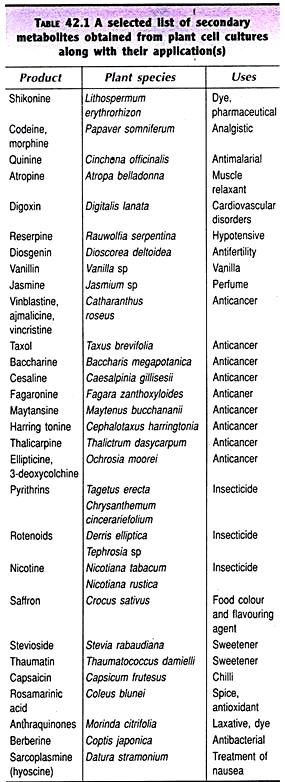
A selected list of plant products obtained from plant cell cultures along with their applications is given in Table 42.1.
Shikonine is a dye produced by the cells Lithospermum erythrorhizon on a commercial scale. The other products successfully produced in plant cell cultures include analgistics (codeine) antimalarial (quinine), muscle relaxants (atropine), drugs to control cardiovascular disorders (digoxin), hypotensives (reserpine), perfumes (jasmine), insecticides (pyrithrins), food sweeteners (stevioside) and anticancer agents (vincristine). Sometimes, the cost of the plant products is unimaginably high. For instance, one kg of vincristine and vinblastine respectively cost $ 3, 500, 00 and $ 1,000,000!
Production of Secondary Metabolites:
The process of in vitro culture of cells for the large scale production of secondary metabolites is complex, and involves the following aspects:
1. Selection of cell lines for high yield of secondary metabolites.
2. Large scale cultivation of plant cells.
3. Medium composition and effect of nutrients.
4. Elicitor-induced production of secondary metabolites.
5. Effect of environmental factors.
6. Biotransformation using plant cell cultures.
7. Secondary metabolite release and analysis.
1. Selection of Cell Lines for High Yield of Secondary Metabolites:
The very purpose of tissue culture is to produce high amounts of secondary metabolites. However, in general, majority of callus and suspension cultures produce less quantities of secondary metabolites. This is mainly due to the lack of fully differentiated cells in the cultures.
Some special techniques have been devised to select cell lines that can produce higher amounts of desired metabolites. These methods are ultimately useful for the separation of producer cells from the non-producer cells. The techniques commonly employed for cell line selection are cell cloning, visual or chemical analysis and selection for resistance.
Cell Cloning:
This is a simple procedure and involves the growth of single cells (taken from a suspension cultures) in a suitable medium. Each cell population is then screened for the secondary metabolite formation. And only those cells with high-yielding ability are selected and maintained by sub-cloning.
Single cell cloning:
There are certain practical difficulties in the isolation and culture of single cells.
Cell aggregate cloning:
Compared to single cell cloning, cell aggregate cloning is much easier, hence preferred by many workers. A schematic representation of cell aggregate cloning for the selection of cells yielding high quantities of secondary metabolites is given in Fig. 42.9. A high yielding plant of the desired metabolite is selected and its explants are first cultured on a solid medium. After establishing the callus cultures, high metabolite producing calluses are identified, and they are grown in suspension cultures.
Cell aggregates from these cultures are grown on solid medium. The freshly developed cell aggregates (calluses) are divided into two parts. One half is grown further, while the other half is used for the quantitative analysis of the desired metabolite produced. The cell lines with high yield of secondary metabolites are selected and used for scale-up in suspension cultures. This is followed by large scale tissue culture in a bioreactor.
Visual or Chemical Analysis:
A direct measurement of some of the secondary metabolites produced by cell lines can be done either by visual or chemical analysis. Visual identification of cell lines producing coloured secondary metabolites (pigments e.g., β-carotene, shikonin) will help in the selection of high-yielding cells. This method is quite simple and non-destructive. The major limitation is that the desired metabolite should be coloured.
Certain secondary metabolites emit fluorescence under UV light, and the corresponding clones can be identified. Some workers use simple, sensitive and inexpensive chemical analytical methods for quantitative estimation of desired metabolites. Analysis is carried out in some colonies derived from single cell cultures. Radioimmunoassay is the most commonly used analytical method. Micro spectrophotometry and fluorescent antibody techniques are also in use.
Selection for Resistance:
Certain cells resistant to toxic compounds may lead to the formation of mutant cells which can overproduce a primary metabolite, and then a secondary metabolite. Such mutants can be selected and used to produce the desired metabolite in large quantities. One example is described.
Cell lines selected for resistance of 5-methyl- tryptophan (analogue of tryptophan) produce strains which can overproduce tryptophan. These tryptophan overproducing strains can synthesize 10-50 times higher levels of the natural auxin namely indole acetic acid (Note: The secondary metabolite indole acetic acid is derived from the primary metabolite tryptophan).
2. Large Scale (Mass) Cultivation of Plant Cells:
In order to achieve industrial production of the desired metabolite, large scale cultivation of plant cells is required. Plant cells (20-150 µm in diameter) are generally 10-100 times larger than bacterial or fungal cell. When cultured, plant cells exhibit changes in volumes and thus variable shapes and sizes. Further, cultured cells have low growth rate and genetic instability. All these aspects have to be considered for mass cultivation of cells.
The following four different culture systems are widely used:
1. Free-cell suspension culture
2. Immobilized cell culture
3. Two-phase system culture
4. Hairy root culture.
Free-cell Suspension Culture:
Mass cultivation of plant cells is most frequently carried out by cell suspension cultures. Care should be taken to achieve good growth rate of cells and efficient formation of the desired secondary metabolite. Many specially designed bioreactors are in use for free-cell suspension cultures.
Some of these are listed below:
i. Batch bioreactors
ii. Continuous bioreactors
iii. Multistage bioreactors
iv. Airlift bioreactors
v. Stirred tank bioreactors.
Two important aspects have to be considered for good success of suspension cultures.
1. Adequate and continuous oxygen supply.
2. Minimal generation of hydrodynamic stresses due to aeration agitation.
Immobilized Cell Cultures:
Plant cells can be made immobile or immovable and used in culture systems. The cells are physically immobilized by entrapment. Besides individual cells, it is also possible to immobilize aggregate cells or even calluses. Homogenous suspensions of cells are most suitable for immobilization.
Surface immobilized plant cell (SIPC) technique efficiently retains the cells and allows them to grow at a higher rate. Further, through immobilization, there is better cell-to-cell contact, and the cells are protected from high liquid shear stresses. All this helps in the maximal production the secondary metabolite.
The common methods adopted for entrapment of cells are briefly described:
1. Entrapment of cells in gels:
The cells or the protoplasts can be entrapped in several gels e.g., alginate, agar, agarose, carrageenin. The gels may be used either individually or in combination. The techniques employed for the immobilization of plant cells are comparable to those used for immobilization of microorganisms or other cells.
2. Entrapment of cells in nets or foams:
Polyurethane foams or nets with various pore sizes are used. The actively growing plant cells in suspension can be immobilized on these foams. The cells divide within the compartments of foam and form aggregates.
3. Entrapment of cells in hollow-fibre membranes:
Tubular hollow fibres composed of cellulose acetate silicone polycarbonate and organized into parallel bundles are used for immobilization of cells. It is possible to entrap cells within and between the fibres. Membrane entrapment is mechanically stable. However, it is more expensive than gel or foam immobilization.
Bioreactors for Use of Immobilized Cells:
Fluidized bed or fixed bed bioreactors are employed to use immobilized cells for large scale cultivation. In the fluidized-bed reactors, the immobilized cells are agitated by a flow of air or by pumping the medium. In contrast, in the fixed-bed bioreactor, the immobilized cells are held stationary (not agitated) and perfused at a slow rate with an aerated culture medium.
Biochemicals produced by using immobilized cells:
A selected list of the immobilized cells from selected plants and their utility to produce important bio-chemicals is given in Table 42.2.
Two-phase System Culture:
Plant cells can be cultivated in an aqueous two phase system for the production of secondary metabolites. In this technique, the cells are kept apart from the product by separation in the bioreactor. This is advantageous since the product can be removed continuously. Certain polymers (e.g., dextran and polyethylene glycol for the separation of phenolic compounds) are used for the separation of phases.
Hairy Root Culture:
Hairy root cultures are used for the production of root-associated metabolites. In general, these cultures have high growth rate and genetic stability. For the production of hairy root cultures, the explant material (plant tissue) is inoculated with the cells of the pathogenic bacterium, Agrobacterium rhizogenes. This organism contains root-inducing (Ri) plasmid that causes genetic transformation of plant tissues, which finally results in hairy root cultures. Hairy roots produced by plant tissues have metabolite features similar to that of normal roots.
Hairy root cultures are most recent organ culture systems and are successfully used for the commercial production of secondary metabolites. A selected list of the plants employed in hairy root cultures and the secondary metabolites produced is given in Table 42.3.
3. Medium Composition and Effect of Nutrients:
The in vitro growth of the plant cells occurs in a suitable medium containing all the requisite elements. The ingredients of the medium effect the growth and metabolism of cells. For optimal production of secondary metabolites, a two-medium approach is desirable.
The first medium is required for good growth of cells (biomass growth) while the second medium, referred to as production medium promotes secondary metabolite formation. The effect of nutrients (carbon and nitrogen sources, phosphate, growth regulators, precursors, vitamins, metal ions) on different species in relation to metabolite formation are variable, some of them are briefly described.
Effect of Carbon Source:
Carbohydrates influence the production of phytochemicals.
Some examples are given below:
1. Increase in sucrose concentration (in the range 4-10%) increases alkaloid production in Catharanthus roseus cultures.
2. Sucrose is a better carbon source than fructose or galactose for diosgenin production by Dioscorea deltoidea or Dalanites aegyptiaca cultures.
3. Low concentration of sucrose increases the production of ubiquinone-10 in tobacco cell cultures.
Effect of Nitrogen Source:
The standard culture media usually contain a mixture of nitrate and ammonia as nitrogen source. Majority of plant cells can tolerate high levels of ammonia. The cultured cells utilize nitrogen for the biosynthesis of amino acids, proteins (including enzymes) and nucleic acids. The nitrogen containing primary metabolites directly influence the secondary metabolites.
In general, high ammonium ion concentrations inhibit secondary metabolite formation while lowering of ammonium nitrogen increases. It is reported that addition of KNO3 and NH4NO3 inhibited anthocyanin (by 90%) and alkaloid (by 80%) production.
Effect of Phosphate:
Inorganic phosphate is essential for photosynthesis and respiration (glycolysis). In addition, many secondary metabolites are produced through phosphorylated intermediates, which subsequently release the phosphate e.g., phenylpropanoids, terpenes, terpenoids. In general, high phosphate levels promote cell growth and primary metabolism while low phosphate concentrations are beneficial for secondary product formation. However, this is not always correct.
Increase in phosphate concentration in the medium may increase, decrease or may not affect product formation e.g.:
1. Increased phosphate concentration increases alkaloid (in Catharanthus roseus), anthraquinone (in Morinda citrifolia) and diosgenin (in Dioscorea deltoidea) production.
2. Decreased phosphate level in the medium increases the formation of anthocyanins and phenolics (in Catharanthus roseus), alkaloids (in Peganum harmala) and solasodine (in Solanum lanciatum).
3. Phosphate concentration (increase or decrease) has no effect on protoberberine (an alkaloid) production by Berberis sp.
Effect of Plant Growth Regulators:
Plant growth regulators (auxins, cytokinins) influence growth, metabolism and differentiation of cultured cells. There are a large number of reports on the influence of growth regulators for the production of secondary metabolites in cultured cells. A few examples are given.
1. Addition of auxins (indole acetic acid, indole pyruvic acid, naphthalene acetic acid) enhanced the production of diosgenin in the cultures of Balanites aegyptiaca.
2. Auxins may inhibit the production of certain secondary metabolites e.g., naphthalene acetic acid and indole acetic acid inhibited the synthesis of anthocyanin in carrot cultures.
3. Another auxin, 2, 4-dichlorophenoxy acetate (2, 4-D) inhibits the production of alkaloids in the cultures of tobacco, and shikonin formation in the cultures of Lithospermum erythrorhizon.
4. Cytokinins promote the production of secondary metabolites in many tissue cultures e.g., ajmalicine in Catharanthus roseus; scopolin and scopoletin in tobacco; carotene in Ricinus sp.
5. In some tissue cultures, cytokinins inhibit product formation e.g., anthroquinones in Morinda citrifolia; shikonin in Lithospermum erythroshizon; nicotine in tobacco.
In actual practice, a combination of auxins and cytokinins is used to achieve maximum production of secondary metabolites in culture systems.
Effect of Precursors:
The substrate molecules that are incorporated into the secondary metabolites are referred to as precursors. In general, addition of precursors to the medium enhances product formation, although they usually inhibit the growth of the culture e.g., alkaloid synthesis in Datura cultures in increased while growth is inhibited by the addition of ornithine, phenylalanine, tyrosine and sodium phenyl pyruvate; precursors tryptamine and secologanin increase ajmalicine production in C. roseus cultures.
4. Elicitor-Induced Production of Secondary Metabolites:
The production of secondary metabolites in plant cultures is generally low and does not meet the commercial demands. There are continuous efforts to understand the mechanism of product formation at the molecular level, and exploit for increased production. The synthesis of majority of secondary metabolites involves multistep reactions and many enzymes. It is possible to stimulate any step to increase product formation.
Elicitors are the compounds of biological origin which stimulate the production of secondary metabolites, and the phenomenon of such stimulation is referred to as elicitation. Elicitors produced within the plant cells are endogenous elicitors e.g., pectin, pectic acid, cellulose, other polysaccharides. When the elicitors are produced by the microorganisms, they are referred to as exogenous elicitors e.g., chitin, chitosan, glucans. All the elicitors of biological origin are biotic elicitors.
The term abiotic elicitors is used to represent the physical (cold, heat, UV light, osmotic pressure) and chemical agents (ethylene, fungicides, antibiotics, salts of heavy metals) that can also increase the product formation. However, the term abiotic stress is used for abiotic elicitors, while elicitors exclusively represent biological compounds.
Phytoalexins:
Plants are capable of defending themselves when attacked by microorganisms, by producing antimicrobial compounds collectively referred to as phytoalexins. Phytoalexins are the chemical weapons of defense against pathogenic microorganisms. Some of the phytoalexins that induce the production of secondary metabolites are regarded as elicitors. Some chemicals can also act as elicitors e.g., actinomycin-D, sodium salt of arachidonic acid, ribonuclease-A, chitosan, poly-L- lysine, nigeran. These compounds are regarded as chemically defined elicitors.
Interactions for Elicitor Formation:
Elicitors are compounds involved in plant- microbe interaction. Three different types of interactions between plants and microorganisms are known that lead to the formation of elicitors.
1. Direct release of elicitor by the microorganisms.
2. Microbial enzymes that can act as elicitors. e.g. endopolygalacturonic acid lyase from Erwinia carotovara.
3. Release of phytoalexins by the action of plant enzymes on cell walls of microorganisms which in turn stimulate formation elicitors from plant cell walls e.g., chitosan from Fusarium cell walls; α-1, 3-endoglucanase from Phytophthora cell walls.
Methodology of Elicitation:
Selection of microorganisms:
A wide range of microorganisms (viruses, bacteria, algae and fungi) that need not be pathogens have been tried in cultures for elicitor induced production of secondary metabolites. Based on the favourable elicitor response, an ideal microorganism is selected. The quantity of the microbial inoculum is important for the formation elicitor.
Co-culture:
Plant cultures (frequently suspension cultures) are inoculated with the selected microorganism to form co-cultures. The cultures are transferred to a fresh medium prior to the inoculation with microorganism. This helps to stimulate the secondary metabolism.
Co-cultures of plant cells with microorganisms may sometimes have inhibitory effect on the plant cells. In such a case, elicitor preparations can be obtained by culturing the selected microorganism on a tissue culture medium, followed by homogenization and autoclaving of the entire culture. This process releases elicitors. In case of heat labile elicitors, the culture homogenate has to be filter sterilized (instead of autoclaving).
In some co-culture systems, direct contact of plant cells and microorganisms can be prevented by immobilization (entrapment) of one of them. In these cultures, plant microbial interaction occurs by diffusion of the elicitor compounds through the medium.
Mechanism of Action of Elicitors:
Elicitors are found to activate genes and increase the synthesis of mRNAs encoding enzymes responsible for the ultimate biosynthesis secondary metabolites. There are some recent reports suggesting the involvement of elicitor mediated calcium-based signal transduction systems that promotes the product formation. When the cells are pretreated with a calcium chelate (EDTA) prior to the addition of elicitor, there occurs a decrease in the production of secondary metabolite.
Elicitor-induced products in cultures:
In Table 42.4, a selected list of elicitor-induced secondary metabolites produced in culture systems are given.
5. Effect of Environmental Factors:
The physical factors namely light, incubation temperature, pH of the medium and aeration of cultures influence the production of secondary metabolites in cultures.
Effect of Light:
Light is absolutely essential for the carbon fixation (photosynthesis) of field-grown plants. Since the carbon fixation is almost absent or very low in plant tissue cultures, light has no effect on the primary metabolism.
However, the light- mediated enzymatic reactions indirectly influence the secondary metabolite formation. The quality of light is also important. Some examples of light- stimulated product formations are given
1. Blue light enhances anthocyanin production in Haplopappus gracilis cell suspensions.
2. White light increases the formation of anthocyanin in the cultures of Catharanthus roseus, Daucus carota and Helianthus tuberosus.
3. White or blue light inhibits naphthoquinone biosynthesis in callus cultures of Lithospermum erythrorhizon.
Effect of Incubation Temperature:
The growth of cultured cells is increased with increase in temperature up to an optimal temperature (25-30°C). However, at least for the production some secondary metabolites lower temperature is advantageous. For instance, in C. roseus cultures, indole alkaloid production is increased by two fold when incubated at 16°C instead of 27°C. Increased temperature was also found to reduce the production of caffeine (by C. sineneis) and nicotine (by N. tabacum).
Effect of pH of the medium:
For good growth of cultures, the pH of the medium is in the range of 5 to 6. There are reports indicating that pH of the medium influences the formation of secondary metabolites. e.g., production of anthocyanin by cultures of Daucus carota was much less when incubated at pH 5.5 than at pH 4.5. This is attributed to the increased degradation of anthocyanin at higher pH.
Aeration of cultures:
Continuous aeration is needed for good growth of cultures, and also for the efficient production of secondary metabolites.
6. Biotransformation Using Plant Cell Cultures:
The conversion of one chemical into another (i.e., a substrate into a final product) by using biological systems (i.e. cell suspensions) as biocatalysts is regarded as biotransformation or bioconversion. The biocatalyst may be free or immobilized, and the process of biotransformation may involve one or more enzymes. Biotransformation involving microorganisms and animal cells are described elsewhere.
The biotechnological application of plant cell cultures in biotransformation reactions involves the conversion of some less important substances to valuable medicinal or commercially important products. In biotransformation, it is necessary to select such cell lines that possess the enzymes for catalysing the desired reactions. Bioconversions may involve many types of reactions e.g., hydroxylation, reduction, glycosylation.
A good example of biotransformation by plant cell cultures is the large scale production of cardiovascular drug digoxin from digitoxin by Digitali lanata. Digoxin production is carried out by immobilized cells of D. lanata in airlift bioreactors. Cell cultures of Digitalis purpurea or Stevia rebaudiana can convert steviol into steviobiocide and steviocide which are 100 times sweeter than cane sugar.
A selected list of biotransformation’s carried out in plant cell cultures is given in Table 42.5.
7. Secondary Metabolite Release and Analysis:
The methods employed for the separation and purification of secondary metabolites from cell cultures are the same as that used for plants.
Sometimes, the products formed within the cells are released into the medium, making the isolation and analysis easy. For the secondary metabolites stored within the vacuoles of cells, two membranes (plasma membrane and tonoplast) have to be disrupted. Permeabilizing agents such as dimethyl sulfoxide (DMSO) can be used for the release of products.
In general, separation and purification of products from plant cell cultures are expensive, therefore every effort is made to make them cost- effective. Two approaches are made in this direction:
1. Production of secondary metabolite should be as high as possible.
2. Formation of side product(s) which interfere with separation must be made minimal.
Once a good quantity of the product is released into the medium, separation and purification techniques (e.g. extraction) can be used for its recovery. These techniques largely depend on the nature of the secondary metabolite.