The following points highlight the four main raw materials used in photosynthesis. The types are: 1. Carbon Dioxide 2. Water 3. Light 4. Chloroplasts.
Raw Material: Type # 1. Carbon Dioxide:
In land plants, carbon dioxide is obtained from the atmosphere through the stomata. Small quantities of carbonates are also absorbed from the soil through the roots. Hydrophytes get their carbon dioxide supply from the aquatic environment as bicarbonates. Bicarbonates are absorbed by the hydrophytes through their general surface.
Experiment 1. Carbon Dioxide is necessary for Photosynthesis — Moll’s Half Leaf Experiment:
Apparatus:
A wide mouthed bottle, Potassium hydroxide solution, cotton, split cork, de-starched potted plant, Vaseline, apparatus for starch test.
Procedure:
De-starch a potted plant by keeping it in dark for 2-3 days. Insert apical half of one leaf in a wide mouthed bottle, containing KOH soaked cotton, by means of split cork. Place the apparatus in sunlight. Provide proper support to the bottle so that the leaf is not pulled. Remove the leaf under study and test for starch as given above.
Observations:
Half of the leaf has turned bluish black. This part was outside the bottle. The apical half which was inside the bottle shows negative starch test.
Inference:
Positive starch test indicates the occurrence of photosynthesis while negative starch test shows absence of photosynthesis. Both the halves of the leaf are receiving light and water.
The apical half of the leaf is deprived of carbon dioxide while the outer half is receiving carbon dioxide from air. Inside the bottle all the carbon dioxide gets dissolved in potassium hydroxide. As the inner half kept in bottle is not performing photosynthesis, it proves that carbon dioxide is necessary for photosynthesis.
Precautions:
(i) Leaf should not touch potassium hydroxide soaked cotton,
(ii) Potassium hydroxide bottle should be provided with proper support,
(iii) Make the connections air tight,
(iv) Handle the leaf carefully during starch test,
(v) Protect the spirit from catching fire through spilling or overheating.
Raw Material: Type # 2. Water:
(i) van Niel (1931), while working on a type of photosynthetic bacteria, found that they required hydrogen sulphide for their carbon fixation. There was no evolution of oxygen. Sulphur globule accumulated as a waste product.
Obviously in these photosynthetic bacteria, carbon dioxide did not split up as there was no evolution of oxygen. Hydrogen sulphide was broken down to provide hydrogen for reduction of carbon dioxide. As a result sulphur accumulates.
6CO2 + 12H2S → C6H12O6 + 6H2O + 12S
From the above observation and conclusions, Van Niel proposed that oxygenic photosynthesis of all organisms is actually an oxidation reduction reaction involving action of hydrogen of water over carbon dioxide to form organic compounds. He also propounded that oxygen is evolved from water.
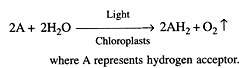
(ii) Robin Hill (1937) illuminated the isolated chloroplasts of Stellaria media in the presence of leaf extract or hydrogen acceptors (e.g., ferricyanides, chromates, benzoquinones, dichlorophenol indophenol, etc.) in the absence of carbon dioxide. The chloroplasts evolved oxygen (Fig. 13.3).
These hydrogen acceptors are also called Hill oxidants while the reaction involving the production of oxygen by the illuminated chloroplasts in the absence of CO2 fixation is called Hill reaction. Vishniac and Ochoa (1951) found that the natural hydrogen acceptor of Hill reaction is NADP+ (nicotinamide adenine dinucleotide phosphate).
(iii) Ruben and Kamen (1941) and Ruben (1941) suspended Chlorella in water having nonradioactive heavy isotope of oxygen, 18O, instead of natural oxygen, 16O. The suspension was illuminated. Oxygen evolved was tested by means of mass spectrometer. It was found to be heavy isotope, 18O. This is possible only if oxygen evolved during photosynthesis comes from splitting of water.
Raw Material: Type # 3. Light:
Light is the visible part of electromagnetic radiations (Fig. 13.4). Electromagnetic radiations are a form of energy that consists of a stream of tiny particles which travel in waves.
Depending upon the wavelength, electromagnetic spectrum consists of 8 types of radiations— cosmic rays, gamma rays, X-rays, ultra-violet radiations, light spectrum, infrared rays, electric rays and radio waves. Visible light consists of radiations having a wavelength between 390-760 nm (or 3900-7600 A).
It can be resolved into light of different colours— violet (390-430 nm), blue or indigo of early workers (430-470 nm), blue-green or blue of early workers (470-500 nm), green (500-580 nm), yellow (580-600 nm), orange (600- 650 nm), orange-red (650-660 nm) and red (660—760 nm).
Red light above 700 nm is called far-red. Radiations shorter than those of violet are called ultra-violet rays. They have a wave length of 100-390 nm.
Similarly, radiations longer than those of red are called infrared. They have wave length of 760-100, 00 nm. Sunlight or solar radiations reaching the earth have wavelength between 300 nm (in the ultraviolet range) to 2600 nm (in the infrared range).
Part of the spectrum used in photosynthesis has a wavelength between 400-700 nm It is called photo-synthetically active radiation (PAR). Leaves appear green because chlorophylls do not absorb green light but allow the same to be reflected and transmitted through leaves.
Blue and red regions of the light spectrum are the most effective in photosynthesis. Blue wavelengths of light carry more energy while red wavelengths have lesser energy.
Therefore, the most efficient wavelengths of light effective in photosynthesis are those of red light. Green light is the least effective in photosynthesis. The light transmitted by the tree canopy is rich in green light. Therefore, plants growing under the canopy of others have lower rates of photosynthesis.
Raw Material: Type # 4. Chloroplasts (Gk. chloros— green, plastos— moulded):
Chloroplasts are green plastids which function as the site of photosynthesis in eukaryotic photoautotrophs. Leaves have the maximum number of chloroplasts with over half a million per square millimetre. Inside the leaves, the chloroplasts occur mostly in the mesophyll cells along their walls for easy diffusion of gases and receiving optimum quantity of incident light.
The chloroplasts align themselves in vertical position along the lateral walls in high light intensity and along tangential walls in moderate light. The change in position helps in receiving optimum light by chloroplasts. A mesophyll cell may have as many as 300 chloroplasts. The latter are found in the peripheral cytoplasm.
A chloropiast is covered by an envelope of two membranes, each of 9-10 nm thickness. They are separated by a translucent zone or periplastidial space of 10-20 nm.
Internally a chloroplast contains two structures, matrix or fluid stroma and membranous system called lamellae or thylakoids. The chloropiast matrix or fluid stroma contains DNA, RNA, ribosomes, enzymes for CO2 assimilation, proteins, starch grains and fat droplets or plastoglobuli.
Chloroplast DNA or ctDNA is naked, circular or linear. It makes the chloroplasts semiautonomous. Ribosomes are of 70S type. With the help of ctDNA, RNA and ribosomes the chloroplasts manufacture some of their own polypeptides, proteins and enzymes.
In the matrix or stroma are embedded a number of flattened membranous sacs called thylakoids or lamellae. Membranes of the thylakoids are also called fret membranes.
They are made up of both proteins and unsaturated lipids roughly in the ratio of 50: 50. Photosynthetic pigments occur partially or completely embedded in the thylakoid membranes. The chlorophylls are associated with both lipids and proteins while carotenoids are associated with lipids only.
At places the thylakoids are aggregated to form stacks of discs called grana. A granum may have 20-50 thylakoid discs. Thylakoids lying outside the grana are called stroma thylakoids or interregnal thylakoids (= lamellae). Grana appear more pigmented because of the grouping of thylakoids. 40-60 grana occur in a chloropiast of Spinach, each with a diameter of 0.3-0.6 mm and a thickness of 0.04-0.08 mm.
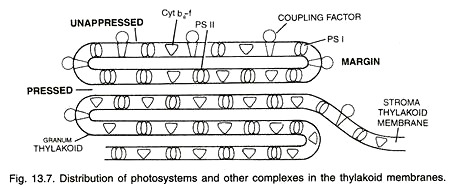
Aggregation of thylakoids in the region of grana is produced by their folding or bifurcations. The space present in the thylakoid of a granum is termed as loculus. The area where thylakoid membranes are appressed together is called partition while their sides are termed as margins.
Thylakoids possess four types of major complexes; photosystem I, photosystem II, Cyt b6-f complex and coupling factor (ATP synthetize). Components of photosystems consisting of reaction centres, antenna pigment molecules and electron transport molecules are associated (mostly non-covalently) with integral membrane proteins.
The proteins project both into the stroma and loculus with hydrophobic amino acids associated with membrane phospholipids. Photosystem II is thought to mostly occur in the appressed or partition regions of granal thylakoids while photosystem I lies in the non-appressed parts as well as stroma thylakoids.
In addition, these parts also possess another complex called coupling factor. It is similar to F0-F1 complex of mitochondria and is called CF0 – CF1. Cooping factor takes part in photophosphorylation. Cyt b6-f complexes are evenly distributed in stroma and granal thylakoids.
A peripheral reticulum of tubules occurs below the chloropiast envelope in C4 chloroplasts.
In photosynthetic prokaryotes (bacteria and cyanobacteria), chloroplasts or equivalent structures are absent. They, however, possess thylakoids. The latter lie freely in the cytoplasm. The pigments are different from those of eukaryotes.
Experiment: Demonstration and Separation of Photosynthetic Pigments:
Apparatus:
Fresh green leaves, acetone, petroleum ether, fine sand, pestle and mortar, a narrow gas jar or a large test tube, test tube stand, dropper, chromatographic paper or Whatman filter paper, paper clip, filtering apparatus, water bath, beaker, measuring cylinder, scissors.
Working:
Take a few fresh green leaves in a mortar. Add a small quantity of fine washed sand and 15-30 ml of acetone. Grind well with the help of pestle and filter the pulp. The filtrate is evaporated over a water bath (40°-50°C) till a small quantity of it is left. It is the pigment concentrate.
Cut a strip of chromatographic paper, narrower than the diameter of a test tube or gas jar. Produce two lateral notches about 2-3 cm from one end. Place a few drops of pigment concentrate one by one on the same spot in the notched part of chromatographic strip and allow it to dry. Connect the other end of the strip with a clip fitted in a cork (a split cork can be used to hold the strip directly).
Pour a small quantity of the solvent (8% acetone + 92% petroleum ether) into the test tube. Hang the chromatographic strip into the test tube in such a way that only its base dips in the solvent. The pigment spot lies about 1 cm above the level of solvent. Fit the cork in the mouth of the test tube.
The solvent is found to move up the strip by capillarity. As it reaches the clip, remove the strip. Allow the strip to dry up by fixing the attached cork to a stand. The dried strip shows four distinct pigment bands, two upper yellowish and two lower greenish (Fig. 13.14).
Results:
The acetone solution contains photosynthetic pigments. When the solvent rises up in the chromatographic strip, it dissolves the pigments and take them to different heights according to their solubility.
The occurrence of four pigment bands shows that photosynthetic pigments are of four types, two yellowish carotenoids and two greenish chlorophylls. The four bands starting from above are orange yellow of carotenes, yellowish of xanthophyll’s, bluish green of chlorophyll a and yellowish green of chlorophyll b.
Precautions:
(i) Take only a few small flush green fresh leaves,
(ii) Evaporate the pigment solution slowly,
(iii) Do not allow the chromatographic strip to touch the walls of the test tube,
(iv) The pigment concentrate should be loaded carefully. It should not spread on the strip,
(v) The pigment spot should not dip in the solvent,
(vi) The cork should be air tight.
Phycobilins:
They are open tetrapyrroles which neither contain magnesium nor phytol. Phycobilins are water soluble. However, they occur in association with proteins or biliproteins. The pigments are of two types— blue (phycocyanin, allo-phycocyanin) and red (phycoerythrin).
The pigments are useful in chromatic adaptations. They are important accessory pigments of blue-green algae, crypto-monads and red algae. In blue-green and red algae, the phycobilins are found inside submicroscopic structures called phycobilisomes attached to thylakoids.
Photosynthetic Units (PSU, Fig. 13.15):
A photosynthetic unit is the smallest group of pigment molecules which take part in a photochemical act or conversion of light energy into chemical energy. It has a photo-centre or reaction centre which is fed by about 200 harvesting pigment molecules. The photo-centre consists of a dimer (Taiz and Zeiger, 2002) of special chlorophyll a molecules, P700 or P680 (named after maximum absorption by pigment or photo-centre).
Reaction centre absorbs light energy at longer wavelengths. The harvesting molecules form a protein based complex called light harvesting complex (LHC). There are distinct LHCs for PS I and PS II. Light harvesting molecules are of two types, antenna molecules and core molecules.
The antenna molecules absorb light of various wavelengths but shorter than that of photo-centre. On absorption of light energy the antenna molecules get excited. In the excited state an electron is pushed to an outer orbital. It lasts for about 10-9 seconds. The excited antenna molecules hand over their energy to core molecules by resonance and come to the ground state.
The energy picked up by core molecules is supplied to the trap or photo-centre. On absorption of energy the photo-centre gets excited and extrudes an electron after which it comes to ground state to repeat the cycle. The frequency of excitation of photo-centre is very high. It cannot be met by its direct absorption of sun energy.
Moreover, the absorption of light of shorter wavelengths cannot be done by it directly. Therefore, photo-centre requires the help of harvesting molecules in the absorption of light energy. Another requirement is the regular supply of electrons from another system.
Photosystems or Pigment Systems (Fig. 13.16):
In green plants, photosynthetic units occur in the form of two distinct groups called photosystems or pigment systems, I and II. They are named after the sequence they were discovered. Each photosystem contains 250-400 pigment molecules.
Photosystem I (PS I):
It is a photosynthetic pigment system along with some electron carriers that is located on both the non-appressed part of grana thylakoids as well as stroma thylakoids. PS I has more of chlorophyll a. Chlorophyll b and carotenoids are comparatively less.
Photosystem I consists of a photo-centre, light harvesting complex (LHC I) and some electron carriers. All are based over membrane proteins. Photo-centre has a dimer of special chlorophyll a molecules called P700. Light harvesting complex has other chlorophyll a molecules, followed by chlorophyll b and carotenoids.
Photosystem I has a reducing agent A0 (which is a special chlorophyll P700 molecule), A1 (a quinone), Fe SX, Fe SA and Fe SB (iron-sulphur proteins), Fd (ferredoxin), cytochrome b6 – f, complex and plastocyanin.
It takes part in both cyclic and noncyclic photophosphorylation. PS I can carry on cyclic photophosphorylation independently. Normally it drives an electron from photosystem II to NADP+ as component of light reaction.
Photosystem II (PS II):
It is a photosynthetic pigment system along with some electron carriers that is located in the appressed part of the grana thylakoids. PS II has chlorophyll a, b and carotenoids. Chi a and chi b contents are equal. Carotenoid content is higher as compared to that of PS I. Photosystem II consists of a photo-centre, oxygen evolving complex, light harvesting complex (LHC II) and some electron carriers.
All are connected to membrane proteins. Photo-centre has a dimer of special chlorophyll a molecules called P680. Light harvesting complex is detachable. It consists of other chlorophyll a molecules, chlorophyll b and carotenoid molecules.
Oxygen evolving complex contains Mn2+, Ca2+ and Сl–. Other components of PS II are phaeophytin, plastoquinone (PQ), cytochrome b6 –f complex and blue coloured copper containing plastocyanin.
It picks up electron released during photolysis of water. The same is extruded on absorption of light energy. As the extruded electron passes over cytochrome b6 – f complex, it energises passage of protons picked up by PQ to create proton gradient for synthesis of ATP from ADP and inorganic phosphate. This photophosphorylation is noncyclic. PS II can operate only in conjunction with PS I.
Anoxygenic photosynthetic bacteria (e.g., Rhodobacter, Rhodopseudomonas) possess a single photosystem where the reaction centre is similar to that of photosystem II.
Electron Transport Chain:
It was first formulated by Hill (1939) while details were worked out later on. Electron transport chain is a series of electron carriers over which electrons pass in a downhill journey releasing energy at every step that is used in generating an electro chemical proton gradient which helps in synthesizing ATP.
Photosynthetic electron transport chain has two components connected with the two photosystems. P680 of photosystem II absorbs light energy, gets excited and transfers its electrons to electron acceptor molecule phaeophytin.
After losing electrons, P680 becomes a strong oxidant, paves the way for light dependent splitting of water called photolysis. It generates electrons which are passed on to electron deficient P680 for performing another photoact. Phaeophytin on accepting electrons becomes strong reducing agent.
It donates its electrons to downstream component of ETC (Q, cyt b – cyt f complex, plastocyanin). Plastocyanin is a copper containing soluble protein which transfers electrons to P700 reaction centre of photosystem I.
On getting excited, P700 hands over electrons to a special chlorophyll molecule X from where electrons are passed to membrane bound iron sulphur proteins (FeS) for transfer to another soluble protein ferredoxin (Fd). The later can pass electrons to reductase complex which helps in reducing NADP+ to NADPH.
This is called Z scheme due to its characteristic zig zag shape. At times when NADP+– reductase complex is not operating, ferredoxin can pass on its electrons to cyt b – cyt f complex for carrying out cyclic photophosphorylation.
Chemiosmotic Hypothesis of ATP Formation:
It was proposed by Mitchell (1961). Electron transport, both in respiration and photosynthesis produces a proton gradient. The gradient develops in the outer chamber or inter-membrane space of mitochondria and inside the thylakoid lumen in chloroplasts.
(i) Lumen of thylakoid becomes enriched with H+ ion due to photolytic splitting of water.
(ii) Primary acceptor of electron is located on the outer side of thylakoid membrane. It transfers its electrons to an H-carrier. The carrier removes a proton from matrix while transporting electron to the inner side of the membrane (Fig. 13.19). The proton is released into the lumen while the electron passes to the next carrier.
(iii) NADP reductase is situated on the outer side of thylakoid membrane. It obtains electron from PS I and protons from matrix to reduce NADP+ to NADP + H+ state.
The consequences of the three events is that concentration of protons decreases in matrix or stroma region while their concentration in thylakoid lumen rises resulting in decrease in pH. A proton gradient develops across the thylakoid.
The proton gradient is broken down due to movement of protons through trans membrane channels, CF0 of ATPase (CF0 – CF1 particle). The rest of the membrane is impermeable to H+. CF0 provides facilitated diffusion to H+ or protons.
As protons move to the other side of ATP, they bring about conformational changes in CF1 particle of ATPase or coupling factor. The transient CF1 particle of ATPase enzyme form ATP from ADP and inorganic phosphate.
Therefore, ATP synthesis through chemiosmosis requires a membrane, a proton pump, a proton gradient and CF0 – CF1 particle or ATPase. Proton pump is energised by electron flow. It creates a proton gradient or high concentration of H+ in the lumen. Protons diffuse across CF0 channels, release energy that activates ATPase enzyme to catalyse ATP (Fig. 13.20). One molecule of ATP is formed when *2H+ pass through ATPase.
Light and Dark Reactions:
Photosynthesis was found quite early to consist of two phases, light and dark. Under normal conditions, the dark phase was found to be rate limiting.
The existence of two phases has come to be known by the following experiments:
1. Flashing Light Experiments:
Warburg (1919) obtained higher rates of photosynthesis in Chlorella when it was exposed to rapid and alternate periods of light and darkness instead of continuous illumination. Emerson and Arnold (1932) found that at 25°C, the maximum photosynthesis took place when light and dark periods were respectively 10-5 sec and 0.055 sec.
At 1.1°C, maximum photosynthesis could be obtained with the same light period but the dark period had to be increased to 0.4 sec. It means that temperature influences reactions of only the dark period which must be purely chemical. Reactions of the light phase are photochemical.
2. Temperature Effects:
At low light intensity, increase in temperature does not increase the rate of photosynthesis. At optimum light intensity and CO2 availability, rate of photosynthesis can be increased or decreased with increase or decrease in temperature.
3. Inhibitors:
Warburg (1920) applied the low concentration of cyanide to the photosynthetic regions. It inhibited photosynthesis under high light intensity more than that at low light intensity.
4. Induction Phase:
When a green plant is suddenly illuminated, there is a gush of oxygen production without simultaneous absorption of CO2.
5. Dark Pick up of Carbon Dioxide:
Carbon dioxide can be fixed in the dark by previously illuminated green cells.