In this essay we will discuss about the Path of Carbon in Photosynthesis. After reading this essay you will learn about: 1. Introduction to Path of Carbon in Photosynthesis 2. C3 Photosynthetic Cycle 3. C4 Photosynthetic Cycle 4. C3 – C4 Intermediates 5. Role of C3 and C4 in Crop Productivity 6. Role of Light in Photosynthesis Control (Metabolic Regulation) and Others.
Contents:
- Essay on the Introduction to Path of Carbon in Photosynthesis
- Essay on the C3 Photosynthetic Cycle
- Essay on the C4 Photosynthetic Cycle
- Essay on the C3 – C4 Intermediates
- Essay on the Role of C3 and C4 in Crop Productivity
- Essay on the Role of Light in Photosynthesis Control (Metabolic Regulation)
- Essay on the Effects of Changing Temperature and Atmospheric Carbon Dioxide Concentration on Photosynthesis
- Essay on the Photosynthetic Carbon Oxidation Cycle
- Essay on the Biochemistry of C2 Cycle
- Essay on the Bacterial Photosynthesis
Contents
- Essay # 1. Introduction to Path of Carbon in Photosynthesis:
- Essay # 2. C3 Photosynthetic Cycle:
- Essay # 3. C4 Photosynthetic Cycle:
- Essay # 4. C3 – C4 Intermediates:
- Essay # 5. Role of C3 and C4 in Crop Productivity:
- Essay # 6. Role of Light in Photosynthesis Control (Metabolic Regulation):
- Essay # 7. Effects of Changing Temperature and Atmospheric Carbon Dioxide Concentration on Photosynthesis:
- Essay # 8. Photosynthetic Carbon Oxidation Cycle:
- Essay # 9. Biochemistry of C2 Cycle:
- Essay # 10. Bacterial Photosynthesis:
Essay # 1. Introduction to Path of Carbon in Photosynthesis:
The path of carbon in photosynthesis includes those reactions which incorporate carbon into more reduced or more energetic compounds. There are three major and one minor pathways by which atmospheric CO2 can be assimilated in photosynthesis. The first is the Calvin cycle or C3 cycle since the early product in this pathway is a C3 compound 3-phosphoglyceric acid (PGA).
It is also named as reductive pentose phosphate pathway or photosynthetic carbon-reduction cycle (PCR cycle). The second is called the C4 cycle because the early products are C4 acids, malate and aspartate, while the terminal steps include reactions of the Calvin cycle.
In the third group, much of the CO2 is fixed in the process known as Crassulacean acid metabolism (CAM), a specialized pattern of photosynthesis in which CO2 is absorbed and stored at night as malic acid and released during the day by decarboxylation inside the tissue in which it is fixed by the Calvin cycle.
This permits water conservation because stomata can remain closed during daytime when there is almost no CO2 assimilation directly from the air.
Intimately connected with and dependent on the C3 cycle, there is another minor cycle named the C2 photo respiratory carbon oxidation cycle or the C2 cycle.
The enzyme responsible for initiating the C3 cycle is ribulose-1, 5-bisphosphate carboxylase (RuBPcase) which has the property for fixing not only CO2 but also O2 leading to the formation of phosphoglycolate and then to glycolate, a C2 compound which is the source of photo respiratory CO2.
Synthesis of sugars from C2 cycle intermediates occurs by the reactions of C3 cycle itself following re-entry of glycolate as PGA of the C3 cycle. Thus it may also be termed as the glycolate pathway or C2 cycle of photosynthesis. It has recently been clarified that the C2 cycle is nothing but an essential and integral part of photosynthesis since three-quarters of the carbon lost as glycolate is returned to the C3 cycle.
Besides the above pathways prevalent in the majority of land plants, another pathway is exhibited by aquatic algae (green microalgae and Cyanobacteria) which possess mechanisms for actively acquiring inorganic carbon (Ci) such as CO2 and HCO3– from the external medium and are able to use this Ci to elevate the CO2 concentration around the active site of Rubisco under extreme CO2 limiting condition.
These plants may be called C-1 plants and the pathway adopted by them C1 pathway, lust like C4 plants where 4-C compound donates CO2 by decarboxylation, 1 -C compound like HCO3– in these C-1 plants donates CO2 for photosynthetic fixation.
From the above different biochemical variants of photosynthetic assimilation of CO2, it is possible to make two important general conclusions:
(i) The C3 cycle is the only known sequence of reactions capable of photosynthetic conversion of CO2 to carbohydrate. One of the important characteristics of the C3 cycle is its autocatalytic ability of generating CO2 acceptor molecule RuBP, while the other cycles do not have this property.
In fact, the other pathways require the operation of several enzymes in addition to those of Calvin cycle, whereas the reactions of Calvin cycle are common to all plants. The Calvin cycle regenerates its own biochemical components that are necessary to maintain the operation of the cycle. The rate of operation of the cycle can be enhanced by increasing the concentration of its intermediates.
The cycle has the feature of producing more substrate than is consumed. If the leaves or isolated chloroplasts kept in dark are illuminated CO2 fixation starts only after a lag period, called the induction period, and the rate of photosynthesis increases with time. This increase is partly due to the activation of enzymes by light and partly due to an increase in the concentration of intermediates of the cycle.
(ii) Other pathways like the C4 and CAM provide a mechanism in plants for concentrating CO2 at the site of RuBP carboxylation and thus improve the CO2-absorbing capacity of the plants. The C2 cycle or glycolate pathway is an inevitable consequence of the C3 cycle reaction and both cycles are integrated together.
Essay # 2. C3 Photosynthetic Cycle:
Calvin cycle was outlined by Calvin and his co-workers (Benson, et al., 1950; Bassham and Calvin, 1957; Calvin and Bassham, 1962; Bassham, 1964) and appears to be acceptable even after four decades. This cycle has been found in all photosynthetic organisms studied so far. The reactions leading to the fixation of CO2 to the level of sugar occur in four distinct phases as shown in Fig. 8.22.
(i) Carboxylation Phase:
This phase consists of the addition of one molecule of CO2 to one 5-carbon sugar, ribulose- 1, 5-bisphosphate (RuBP) with the formation of two molecules of PGA. The reaction is catalyzed by the enzyme ribulose bisphosphate carboxylase which also functions as an oxygenase.
(a) Ribulose-1, 5-Bisphosphate Carboxylase-Oxygenase (RubisCO):
The first step in Calvin cycle is the ribulose-1, 5-bisphosphate carboxylase (RuBPcase) reaction in which CO2 and H2O are combined with ribulose-1, 5-bisphosphate (RuBP).
It has been postulated that the immediate product of the carboxylation of RuBP is an unstable C6 intermediate, 2-carboxy-3-keto-D-ribitol 1, 5-bisphosphate which is immediately hydrolyzed to two molecules of 3-PGA. This reaction involves the addition of one carbon molecule (CO2) to a 5- carbon acceptor (C5) to form two C3 molecules.
It is now realized that O2 can compete with CO2 for the same site on the enzyme, giving rise to the oxygenase mode of action of the same enzyme (RuBP oxygenase) which yields one PGA (C3) molecule together with a C2 product, phosphoglycolate.
This RuBP oxygenase reaction is the basis of the photo respiratory carbon oxidation cycle. Thus, when CO2 is high and O2 is less, the enzyme shows carboxylase reaction. Alternatively, when O2 is high and CO2 is low, the same enzyme shows oxygenase activity.
Comparison of RuBP carboxylase and RuBP oxygenase action:
RuBPcase was previously called carboxydismutase because it catalyzes not only a carboxylase reaction but also an intermolecular oxidation-reduction reaction called a dismutation. RuBisCO may comprise up to half of the soluble protein in leaves and localized exclusively within the chloroplasts. This single protein can be distinguished from the remaining leaf protein by ultracentrifugation — called Fraction I protein.
This protein is large, (MW 550,000) and highly oligomeric consisting of eight large subunits (MW 55,000) and eight small subunits (MW 15,000). It is relatively inefficient as a catalyst. Under in vitro conditions, the Km for CO2 is very high (200 – 500 µM), while the Km under in vivo condition is about 15 µM.
The synthesis of this vital and abundant protein i.e. RuBisCO or fraction I protein requires the close cooperation and coordination of both chloroplast and nuclear genomes and of both chloroplast and cytoplasmic ribosomes.
The large subunit is both encoded and synthesized within the chloroplast, while the small sub-unites both encoded and synthesized outside the chloroplast which is subsequently transferred into the chloroplast.
Cytoplasmic ribosomes (80S) make the small subunit of fraction I protein, while chloroplast ribosomes (70S) make the large subunit. It has been suggested that the small subunit acts as a positive factor required for the initiation of the translation of the mRNA for the large subunit, implying that the nuclear genome is controlling the overall rate of synthesis of fraction I protein.
The assemblage of both subunits into the active enzyme takes place in the chloroplast. Thus there is a close inter-genomic cooperation and interaction between an organelle and a nucleocytoplasmic system.
(ii) Reduction Phase:
The following two reactions together constitute the reduction phase. PGA formed in the previous carboxylation phase is phosphorylated by ATP to form 1, 3-bisphosphoglycerate (BPGA). This reaction is catalysed by 3-phosphoglycerate kinase. It is to be noted that the same enzyme operates in the glycolate pathway in the cytosol in the reverse direction to form ATP.
In the second reaction, the phosphorylated PGA (BPGA) undergoes actual reduction to glyceraldehyde phosphate, catalyzed by an NADP-dependent glyceraldehyde-3-phosphate dehydrogenase located in the chloroplast. In glycolysis, the reverse reaction is catalyzed by an enzyme using NAD+ as the cofactor.
The formation of glyceraldehyde-3-phosphate is the only reductive step in Calvin cycle and it is also the principal site of Pi release. The enzyme is activated by light since illumination of chloroplasts or leaves before enzyme extraction has been found to increase the activity.
(iii) Regeneration Phase:
Several reactions in this phase act to regenerate the CO2 acceptor RuBP and ensure the continuation of the cycle. Glyceraldehyde phosphate and its isomer dihydroxyacetone phosphate (DHAP) are collectively known as triose phosphates. DHAP is produced from glyceraldehyde-3-phosphate by triose phosphate isomerase.
There are two aldolase reactions in the pathway. In the first aldolase reaction, called fructose- 1,6-bisphosphate aldolase, glyceraldehyde 3-P is combined with DHAP to form the 6-carbon molecule fructose-1, 6-bisphosphate.
In the next reaction, the hydrolysis of fructose-1, 6-P2 to fructose-6-phosphate takes place which is the second phosphate releasing step of the cycle. This is catalysed by a specific fructose-1, 6-bisphosphatase confined to chloroplasts that is different from the cytoplasmic enzyme involved in the glycolytic pathway. This enzyme is also activated in illuminated chloroplasts.
Then there are two transketolase reactions. This enzyme catalyzes the transfer of a C2 fragment from a ketol donor to an acceptor aldehyde and thiamine pyrophosphate (TPP) and Mg2 + remain bound to the enzyme.
The enzyme is not specific for fructose-6-P and sedoheptulose-7-P may also act as the ketol donor as shown in the second transketolase reaction. It was previously thought that the C2 fragment transferred from the ketol donor gives rise to glycolate, but at present this view is not accepted.
The next step is the second aldolase reaction similar to the formation of fructose-1, 6-P2 . Here, a 7-carbon bisphosphate, that is, sedoheptulose-1, 7-bisphosphate is formed by the combination of erthyrose-4-P (C4) with DHAP-the enzyme is sedoheptulose-1, 7- bisphosphate aldolase. 
Then sedoheptulose-1, 7-P2 is hydrolyzed to its monophosphate, sedoheptulose-7-phosphate and Pi is released. This step is catalyzed by sedoheptulose-1, 7-bisphosphatase which is analogous to the fructose-1, 6-P2ase reaction where fructose bisphosphate is hydrolyzed. The chloroplast sedoheptulose-1, 7-P2ase is activated by light.
This is followed by the second transketolase reaction in which the C2 fragment is removed from the C7 sugar sedoheptulose-1, 7-P2 leading to the formation of residual C5 sugar aldehyde (ribose-5- P) and a C5 keto sugar (xylulose-5-P).
In the next reaction, xylulose-5-P is converted to ribulose-5-P by ribulose-5-P epimerase. This enzyme catalyzes an isomerization reaction in which two epimers are involved having different orientations of the H and OH groups on C-3.
Ribose 5-P formed in the previous transketolase reaction is converted P by enzyme ribose 5-phosphate isomerase but here the isomerization ketopentose and an aldopentose.
The regeneration cycle is completed by the phosphorylation of ribulose -5-P and thus the CO2 acceptor ribulose-1, 5-P2 (RuBP) is regenerated. This step is catalyzed by phosphoribulokinase in which ATP acts as the Pi donor and the enzyme is activated by light.
(iv) Product Synthesis Phase:
Carbohydrates like sucrose and starch are the primary end products of photosynthesis. In addition, fats, fatty acids, organic acids and amino acids have also been shown to be formed as secondary products of photosynthetic CO2 fixation under different environmental condition of light, CO2 and O2.
Lower groups of plants, particularly algae, produce starch-like polysaccharides and a number of soluble carbohydrates. In red and blue-green algae, floridean starch is formed which are polymers of glucose-with branched chains, whereas starch of higher plants has straight chains of α-1, 4- glucans.
Another difference is that floridean starch is synthesized outside the chloroplasts and not in the chloroplasts as higher plant starch.
Soluble products of photosynthesis like mannitol, a derivative of mannose is produced mainly in brown algae and dino-flagellates. In brown algae, mannitol acts as a substrate both of respiration and for laminarin synthesis. Green algae produce sucrose whereas red algae produce galactosides and sorbitol but these are not converted to polysaccharides.
The net result of the light reaction and the Calvin cycle reactions is the conversion of CO2, H2O and inorganic phosphate to triose phosphates. The last step (i.e., product synthesis phase) in photosynthetic carbon metabolism is, therefore, the conversion of triose phosphate to carbohydrate end product.
This process releases inorganic phosphate (Pi), which can be used for further ATP synthesis, and thus is a prerequisite for further CO2 fixation.
Sucrose is synthesized in the cytosol from triose phosphates, which exit Calvin cycle before the plastid fructose 1, 6-bisphosphatase (pFBPase). There is a strict coupling of triose phosphate export and phosphate uptake because it is facilitated by triose phosphate trans-locator (TPT), which catalyzes a strict counter-exchange.
Starch is synthesized in plastid from fructose 6-phosphate, which is withdrawn from the regeneration phase of Calvin cycle between the plastid fructose 1, 6-bisphosphatase (pFBPase) and sedoheptulose 1, 7-bisphosphatase (SBPase). Thus carbohydrate like sucrose and starch are the primary end products of photosynthesis.
Sucrose is synthesized in the cytoplasm from triose phosphates (dihydroxyacetone phosphate and glyceraldehyde-3-P) exported from the chloroplasts, via the chloroplast envelope in exchange for orthophosphate (Pi).
In the cytoplasm, these two triose phosphates combine to form fructose-1, 6-bisphosphate (an aldolase reaction) which undergoes hydrolysis to form fructose-6-phosphate by the enzyme fructose -1, 6-bisphosphatase.
Nucleoside di-phosphate derivatives of glucose are the precursors for sucrose synthesis and are produced in cytoplasm by the following steps:
The main pathway of sucrose synthesis in photosynthetic tissues is supported by sucrose phosphate synthetase and sucrose phosphorylase:
In some plants, the following pathway may exist in which fructose itself and not fructose-6-P can be utilized as the substrate in a reaction catalysed by sucrose synthetase:
It is now believed that the main, function of sucrose synthetase seems to be the formation of UDP-glucose from sucrose by supporting the reaction in the opposite direction which is related to starch synthesis.
When CO2 fixation rate in chloroplasts is quite vigorous, the rate of accumulation of triose phosphates there exceeds the rate of their transport to the cytoplasm for being converted to sucrose. Synthesis of starch (α-1, 4-glucan) occurs in the chloroplast stroma.
The triose phosphates are converted to glucose 1 -P which then reacts with ATP as:
This reactions catalyzed by the enzyme ADP-glucose pyrophosphorylase which synthesizes ADP-glucose, the substrate for starch (α-1, 4-glucan) synthetase as shown below:
A small amount of starch shown as glucosen in the above equation actually acts as a primer to which molecules of glucose are added successively (glucosen+1) thus increasing the size of the starch molecule by chain elongation. Starch breakdown is mediated by phosphorylase or amylase to produce sugar phosphates or sugars followed by glycolytic reactions to yield PGA for being utilized by the plant during darkness.
Since triose phosphates are partitioned between sucrose synthesis in the cytoplasm and starch formation in the chloroplasts and because chloroplasts normally make starch in light and degrade it in darkness, some control mechanism for these activities is expected to exist.
A major controlling factor is ADP-glucose phosphorylase which is activated by PGA and inhibited by Pi, thus the rate of starch synthesis is dependent on PGA/Pi ratio in the stroma.
Another factor controlling sucrose biosynthesis is the Pi liberated during sucrose phosphorylase reaction in the cytoplasm and this Pi must be transported to the chloroplast by the activity of phosphate trans-locator present in the chloroplast envelope which exchanges Pi and triose phosphates across the chloroplast membrane.
Another control of sucrose synthesis is exerted by the regulation of fructose bisphosphatase activity, which is inhibited by fructose-2, 6-P2 present in cytoplasm.
Essay # 3. C4 Photosynthetic Cycle:
A major new pathway for carbon flow during photosynthesis other than Calvin cycle was established in sugarcane, maize, sorghum and related grasses. Initial studies by Kaprilov (1960) in Russia on maize and by Kortschak, Hart and Burr (1965) in Hawaii on sugarcane revealed that the major early products of photosynthesizing leaves exposed to 14CO2 are the C4 acids malate and aspartate.
Hatch and Slack (1966) in Australia confirmed the earlier observation and proposed a cyclic reaction mechanism in which a C3 acid is carboxylated (first carboxylation) to yield a C4 acid and that subsequently donates one carbon as CO2 to the reductive photosynthetic cycle or Calvin cycle where the second carboxylation takes place.
The new pathway was originally named as C4– dicarboxylic acid pathway. It is now referred to as the C4 pathway and the plants possessing this pathway are termed C4 plants.
Thus, C4 plants can be defined on the basis of the following characters:
1. Primary initial products of CO2 fixation are the 4-carbon dicarboxylic acids oxaloacetate, malate and aspartate. Hence, the name C4 has been given in comparison to C3 plants where the initial products of CO2 fixation is the 3-carbon acid, 3-phosphoglyceric acid.
2. CO2 fixation into C4 acids occurs in the light and not in darkness like CAM-plants.
3. Carbon is donated from the C4 acids into the C3 cycle, i.e., C-4 of C4 acid to C-1 of 3- phosphoglycerate.
4. Plants possess Kranz anatomy, i.e., there are two distinct cell types, outer mesophyll cells and inner bundle sheath cells occurring as concentric rings. C4 acids are formed in the outer mesophyll cell layer by primary carbon assimilation whereas carbon reduction to the level of carbohydrates takes place in the inner bundle sheath layer (Kranz layer).
A simplified scheme, as shown in Fig. 8.24 shows that the primary assimilation of CO2 occurs in mesophyll cells with phosphoenolpyruvate (PEP) as CO2 acceptor and by the enzyme phosphoenolpyruvate carboxylase (PEPcase) C4 acid oxaloacetic acid is produced.
This unstable first product of carboxylation is converted to a more stable C4 acid, either malate or aspartate that is transported to the bundle sheath cells of the leaf.
The C4 acid is then decarboxylated and CO2 thus produced is re-fixed by RuBPcase in the conventional C3 cycle reactions in bundle sheath cells.
The C3 acid remaining (either pyruvate or alanine formed from it) after C4 acid decarboxylation diffuses back into the mesophyll cells where it is converted to PEP by the enzyme pyruvate orthophosphate dikinase, thus regenerating the CO2 acceptor. The last step is critical for the continued operation of the process.
Unique Importance of Rubisco:
Rubisco catalyzes the key reaction in which RuBP acts as acceptor for CO2 forming two molecules of PGA. The competing reaction with O2 leads to the formation of phosphoglycolate, which is recycled via photo respiratory pathway. Because of the relatively high Km for CO2 and the competition with O2 Rubisco activity is limited by the CO2 level in ambient conditions.
The relative inefficiency of the enzyme is partly counterbalanced by the very high amount constituting up to 30% of protein in the leaf. Relatively poor affinity for CO2 also requires a high stomatal conductance during rapid photosynthesis. Entry of CO2 will undoubtedly be facilitated with stomata remaining wide open, which will result in increased transpiration.
This is a problem for terrestrial plants having a limited water supply. Another property of this enzyme is that a large number of phosphorylated compounds may bind to the active site of Rubisco, thereby leading to a competition with RuBP.
(a) Regulation of Rubisco:
(i) Carbamylation (Activation) State of Rubisco:
Rubisco enzyme is unique because the catalytic site of the active enzyme contains a carbamylated lysine to which a Mg2+ is complexed. The activated state of the enzyme is termed ECM form of enzyme. The enzyme can be carbamylated or de-carbamylated in vivo, leading to a change in activation state ECM/(E + ECM).
Activation state of Rubisco increases with increasing irradiance. A protein called Rubisco activase is needed to cause this activation. The activase enzyme operates by decreasing the affinity of de-carbamylated enzyme for various inhibitory sugar bisphosphates (usually, FBP and SBP), which would otherwise occupy the inactive or decarbamylated form and inhibit carbamylation or catalysis.
It has been shown that the inhibitory sugar bisphosphates include RuBP itself which binds tightly to the de-carbamylated form of Rubisco. The activase appears to be involved in removing RuBP from the active site to allow carbamylation. The activation of Rubiso is regulated to achieve a balance between RuBP formation and its consumption, so that the active Rubisco sites (ECM) are almost saturated by RuBP.
(ii) Rubisco Regulation by 2-Carboxyarabinitol-1 –Phosphate:
Leaves of some plants, such as soybean, contain an unusual sugar phosphate, 2-carboxyarabinitol-1 -phosphate (CAP), which is an analog of the transition state intermediate of carboxylation reaction, i.e., 2-carboxy-3-keto-arabinitol 1, 5-bisphosphate.
It was found that Rubisco extracted from darkened leaves could not be activated by CO2 and Mg2+, whereas Rubisco isolated from light-exposed leaves could readily be activated.
It was shown that this was due to the presence of an inhibitor CAP which binds with high affinity to the activated (ECM) form of Rubisco and depresses Vmax. Rubisco activase may also be involved in removing bound CAP from the enzyme.
Although CAP seems to be present in all plants, it can only play a role in regulation of Rubisco when present in large amount as in legumes like bean, soybean, tomato and sunflower. It is also present in small amounts in leaves of plants such as spinach, wheat or maize. In species which contain large amounts of CAP the concentration increases in dark and decreases in light.
These leaves contain specific CAP phosphatase which converts CAP into carboxyarabinitol (CA). In fact, leaves contain considerable amount of free sugar (CA) in both light and dark which does not have inhibitory property. CA can act as a precursor of inhibitor CAP in dark and CAP is converted back to CA after illumination, suggesting the existence of a metabolic cycle between the two reactions.
Structure related to CAP is 2-carboxy-3-ketoarabinitol-1, 5-bisphosphate which is the transition state analog of RuBP carboxylase reaction. CAP binds with high affinity to ECM (active) form of Rubisco and inhibits it.
(iii) Regulation of Rubisco Gene Expression by Phytochrome:
The chloroplast proteins like the small subunit (SSU) of Rubisco and the major light harvesting chlorophyll protein (LHCP) associated with light harvesting complex of PSII are synthesized in response to light stimulus. The nuclear genes encoding messages for these two proteins are regulated by light. These two proteins play an important role in chloroplast development and greening.
The genes for these proteins — rbcS and cab respectively are present in multiple copies in the genome, i.e., they belong to multi-gene families. Red light causes conversion of Pr to Pfr. Pfr activates regulatory proteins which bind to light regulated elements (LRE) and stimulate transcription of the nuclear genes.
i. Reactions of the C4Photosynthesis.
(i) Reactions in the Mesophyll Cells:
(a) Carboxylation Reaction:
The primary carboxylation of the C4 cycle is done by PEP carboxylase using HCO3– as the substrate to yield oxaloacetic acid. The activity of the enzyme carbonic anhydrase seems to be intimately associated with photosynthesis because it catalyzes the hydration of CO2 forming HCO3–, the substrate for PEP case as;
PEPcase is located in the cytoplasm of mesophyll cells and is activated by Mg2+ and is inhibited by malate and aspartate through feedback control.
The product of carboxylation, oxaloacetic acid is reduced to malate in mesophyll chloroplasts of C4 leaves of NADP-ME type by enzyme NADP malate dehydrogenase and malate is transferred to bundle sheath cells.
In PCK or NAD-ME types, oxaloacetic acid is converted to aspartate by the enzyme aspartate aminotransferase located in the cytoplasm of mesophyll cells and the resulting aspartate is transferred to bundle sheath cells.
(b) Formation of PEP:
In the chloroplasts of mesophyll cells, a key enzyme pyruvate orthophosphate (Pi) dikinase affords phosphorylation of pyruvate towards the synthesis of PEP, the substrate for C4 carboxylation at the expense of photo synthetically derived ATP, while Pi is the other substrate being phosphorylated to pyrophosphate (PPi).
This activity is intimately linked with two other mesophyll cell chloroplast enzymes, adenylate kinase and pyro-phosphatase which jointly act to provide ADP and Pi as the products presumably for use in ATP synthesis.
The above three enzymatic reactions may be summarized in the following equation:
It should be noted that the formation of PEP from pyruvate via other means either by pyruvate kinase or by PEP synthetase is thermodynamically not favourable for PEP synthesis because the equilibrium is towards PEP breakdown.
However, since pyruvate Pi dikinase is coupled with adenylate kinase and pyro-phosphatase, PEP synthesis is favoured by the energy of hydrolysis of an additional ATP.
(c) Reduction of PGA:
Although lacking in RuBP case, mesophyll cell chloroplasts are functionally unique in the sense that these contain the enzymes for reducing PGA to triose phosphate. It is likely that a portion of PGA formed in bundle sheath chloroplasts may move to mesophyll chloroplasts, where it is reduced to triose phosphate by the available photo-generated NADPH.
This is particularly true in PCK and NAD-ME plants that transport aspartate. Thus, the photo-generated NADPH not being consumed in oxaloacetate-malate conversion can be utilized in PGA reduction. Mesophyll cell chloroplasts, however, lack RuBPcase.
Triose phosphate resulting from PGA reduction may be transferred to bundle sheath chloroplasts and used for starch and sucrose synthesis via the C3 cycle. It also seems probable that much of the PGA exported from bundle sheath chloroplasts of C4 plants is used for sucrose and starch synthesis in mesophyll cells since the necessary enzymes for such conversion have been reported to be present there.
ii. Reactions of Bundle Sheath Cells:
Decarboxylation Mechanisms:
There are three C4 subgroups according to the different mechanisms by which decarboxylation takes place in bundle sheath cells.
(a) NADP-Malic Enzyme (NADP-ME) Type:
Here C4 malate is transferred from mesophyll to bundle sheath cells and C3 pyruvate is returned. Malate is decarboxylated in bundle sheath chloroplasts via an NADP-specific malic enzyme.
CO2 so formed is fixed by the C3 cycle. NADPH formed in this reaction is recycled by coupling to the reducing step requiring NADPH of the C3 cycle. Such coupling serves to provide half of the total NADPH necessary for reducing two molecules of PGA resulting from CO2 fixation.
(b) PEP-Carboxykinase (PEP-CK or PCK) Type:
Remaining C4 plants contain little NADP malic enzyme but high amounts of aspartate aminotransferase and alanine aminotransferase, distributed equally between mesophyll and bundle sheath cells. Aspartate transported from mesophyll cells is first converted back to oxaloacetate in bundle sheath cell cytoplasm by aspartate aminotransferase.
The subsequent fate of this oxaloacetate will, however, vary in different groups of C4 plants according to two mechanisms of its decarboxylation. In one group, oxaloacetate is directly decarboxylated by the enzyme PEP carboxykinase in bundle sheath chloroplast to provide CO2 for assimilation by C3 cycle.
It has been proposed that PEP, the product of oxaloacetate decarboxylation moves across the chloroplast into the cytoplasm of bundle sheath cells and gives rise to pyruvate by pyruvate kinase which is then converted to alanine by alanine aminotransferase and that alanine is returned to mesophyll cells.
The latter aminotransferase reaction is significant since it provides the necessary amino group coupling with the other aminotransferase, aspartic aminotransferase and finally the return of alanine from the bundle sheath to mesophyll would maintain the balance of amino acids between the two cell types. Plants utilizing this mechanism of C4 acid decarboxylation have been designated as PCK-type.
(c) NAD-Malic Enzyme (NAD-ME) Type:
Like PCK type, this group is also characterized by the transfer of aspartate and return of alanine, but here the conversion of aspartate to oxaloacetate and its decarboxylation to pyruvate occur inside mitochondria. Although this group of plants contain high aminotransferase activities to support keto acid – amino acid inter-conversions, they lack PEP carboxykinase.
So, a direct decarboxylation of oxaloacetate to PEP is not possible here, instead oxaloacetate which is produced from aspartate in bundle sheath mitochondria via aspartate aminotransferase activity is then reduced to malate by NAD malate dehydrogenase located in the mitochondria.
This malate is next decarboxylated by NAD-malic enzyme (hence the name NAD-ME) whereas NAD-NADH cycle couples the two latter reactions.
Pyruvate moving from mitochondria is then converted to alanine in cytoplasm via alanine aminotransferase. Here coupling of this reaction with aspartic aminotransferase would involve the movement of α-ketoglutarate and glutamate, the amino group acceptor and donor, between mitochondria and cytoplasm.
Re-Fixation of CO2 by C3 Cycle:
Bundle sheath chloroplasts contain all the enzymes of C3 cycle including RuBPcase. The CO2 liberated by C4 acid decarboxylation via any of the three pathways is ultimately used in carbohydrate synthesis.
Anatomical Characteristics of C4 Photosynthesis:
(a) Kranz Anatomy:
Plants showing C4 cycle also have a specialized leaf anatomy, known as Kranz anatomy (Kranz = halo, wreath or ring). Here, the vascular bundles are surrounded by the bundle sheath forming a ring (Kranz) of cells and in contrast to the C3 plants, these cells are thick-walled, radially arranged and highly developed containing specialized chloroplasts.
This ring of bundle sheath cells is in turn surrounded by one layer of chloroplast containing mesophyll cells which are more dense and compact than in C3 leaves.
There is a possible division of labour between chloroplasts of these two cell types since initial fixation of CO2 occurs in the mesophyll cells and the products are transported to bundle sheath chloroplasts where these are converted to starch and eventually trans-located to a carbon -sink.
Kranz anatomy is correlated with high rates of assimilation and retention of carbon and also rapid transport of photosynthates from bundle sheath to phloem.
(b) Chloroplast Dimorphism:
Pronounced structural dimorphism and sometimes size dimorphism of chloroplasts is a feature of all C4 plants.
Laetsh (1974) analysed the chloroplasts of C4 plants and showed that the morphological structure and size differ in chloroplasts in these two cell types, i.e., mesophyll and bundle sheath cells. Mesophyll chloroplasts are small and usually do not contain starch while bundle sheath chloroplasts are large and contain starch.
Mesophyll chloroplasts have well-developed grana whereas bundle sheath chloroplasts are either usually agranal or possess rudimentary grana. Those C4 plants which transport malate (NADP-ME type) specifically lack grana.
It has been suggested that PS II activity is absent in agranal chloroplast and that the reducing power (NADPH) for C3 cycle operation is generated in bundle sheath chloroplasts by decarboxylation of malate by NADP-malic enzyme.
It should be recognized that mesophyll chloroplasts are also capable of forming starch but starch accumulation there is actually governed by a source-sink relationship maintaining a gradient from mesophyll to bundle sheath and then to phloem.
(c) Structural Basis for Function:
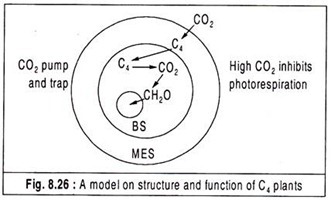
A currently accepted model on structure and function of the anatomical features of C4 plants is presented in Fig. 8.26.
An important principle of this model is that CO2 is concentrated in the bundle sheath cells as a result of transport of malate and aspartate from the mesophyll cells. This CO2 pump which operates in mesophyll cells provides high CO2 level in bundle sheath cells and supports the RuBPcase in its carboxylase mode of action and this inhibits the production of glycolate and hence photorespiration.
Kranz anatomy is thus an adaptation which achieves a CO2 concentration in the internal tissues which inhibits photorespiration and subsequent loss of CO2. Furthermore, less O2 concentration is likely to be associated with relatively less PS II activity of bundle sheath chloroplasts lacking well- developed grana and this situation seems to minimize O2 inhibition.
Systematics, Evolution and Genetics of C4 Photosynthesis:
So far, C4 photosynthesis has been reported in 18 taxonomically diverse families (3 monocots and 15 dicots), 196 genera and 943 species. A list of families containing C4 plants is given in Table 8.2.
No algae, bryophyta, lower vascular plants, gymnosperms and primitive angiosperms have been reported to have C4 activity. From this taxonomic distribution, it is pertinent to suggest that C4 pathway is a derived condition having evolved polyphyletically among more advanced orders of C3 monocots and dicots.
The family Poaceae (Graminae) which contains most of the C4 species is interesting because C4 condition appears in most of the species in the subfamilies Panicoideae and Eragrostoideae but in no species of Festucoideae, Arundinoideae, Oryzoideae and Bambusoideae.
Furthermore, all three types of C4 cycle are found in Panicoideae whereas Eragrostoideae are either PCK of NAD-ME. It has been found that both C3 and C4 plants exist in the same family or genus. In the genus Atriplex, some species are C3 and some are C4.
Maize, sugarcane and sorghum are the known major crop species with C4 pathway. Therefore, from the standpoint of crop productivity, it would be desirable to breed C4 cycle into C3 stock. Bjorkman and his associates in 1976 have reported about successful hybridization between related C3 and C4 species of Atriplex and one such cross between. A. rosea (C4) and A. patula (C3) has been followed for many generations.
A number of hybrids having various C3 – C4 intermediate characters have been produced and several hybrids resemble their C4 parent both in leaf anatomy and in certain biochemical traits like high PEPcase but none of the hybrids is a completely functional C4 plant. They concluded that genetic diversity between C3 and C4 is not great and is controlled by only a small number of genes.
These results suggest that the presence of the necessary components of C4 is not enough, rather an integrated operation of the C4 pathway together with proper co-ordination and spatial compartmentation are also required. Thus, it is extremely difficult to introduce C4 characters into agriculturally important C3 species through genetic manipulation.
There are, however, certain plants which appear to be intermediate in character between C3 and C4 that means they possess an imperfect C4 cycle. Kennedy and his co-workers in 1977 have shown that Mollugo verticillata belonging to Aizoaceae is a C3 – C4 intermediate.
Similarly, species of Moricandia show various C3 – C4 intermediate anatomical and biochemical characters (Apel and Ohle, 1979). In Panicum milioides, the main fixation pathway is C3, but an adaptive PEPcase is able to increase the internal CO2 level to some extent, thus reducing photorespiration (Rathnam and Collet, 1978, 1979).
A peculiar feature of C4 plants is that they require small amounts of sodium ions for normal growth (Brownell and Crossland, 1972). The exact biochemical site of action of Na+ required as a micronutrient has not been elucidated, it is probably required for some metabolic reaction specific for C4 pathway.
Essay # 4. C3 – C4 Intermediates:
More than 20 plant species exhibit photosynthetic traits that are intermediate between C3 and C4 plants. These are species belonging to the genera Alternanthera, Flaveria, Neurachne, Moricandia, Panicum and Parthenium. These show reduced photorespiration and low CO2 compensation point, typical of C4 plants. But at the same time, they have a weakly developed Kranz anatomy, compared with true C4 species.
In contrast to either C3 or C4 plants, Rubisco in these plants is located both in mesophyll and bundle sheath cells.
Furthermore, the activity of key enzymes of C4 pathway, viz., pyruvate Pi dikinase, PEP carboxylase, NAD-malic enzyme, NADP-malic enzyme and PEP- carboxykinase, is very low, and they do not have a functional C4-acid cycle. It has been observed that their low CO2 compensation point is due to light-dependent recapture by mesophyll cells of CO2 released in bundle sheath cells in photorespiration.
The bundle sheath cells of C3-C4 intermediates contain a large fraction of organelles involved in photorespiration.
These C3-C4 intermediates have a system to scavenge photo respiratory CO2 that escapes from bundle sheath cells but they do not have CO2 concentrating mechanism of true C4 species. Glycine decarboxylase, which is the key enzyme of photorespiration that releases photo respiratory CO2, occurs exclusively in cells that surround vascular bundle, i.e., bundle sheath cells.
In contrast, the mitochondria of the mesophyll cells, like those of true C4 plants, have a low activity of glycine decarboxylase. It is possible that the products of RuBP oxygenase reaction including glycine move to the bundle sheath cells. The products are metabolized in the bundle sheath and, therefore, serine cannot move back to mesophyll.
Since glycine carboxylase is located only in the bundle sheath cells, CO2 released in photorespiration is likely to enrich the environment of Rubisco which is also present in bundle sheath. Thus it appears that these intermediates seem to adopt a CO2 concentrating mechanism of different nature, although not to the same extent as in true C4 plants.
The location of glycine decarboxylase provides another advantage, because it increases the diffusion path for CO2 between the site of release, i.e., bundle sheath cells, and the atmosphere. This allows the recapture of photo respiratory CO2 by Rubisco, located in both bundle sheath and mesophyll cells.
Significance of C4 Cycle:
The essential function of carboxylation followed by decarboxylation employed in the C4 pathway is to increase the CO2 concentration at the site of C3 photosynthesis in bundle sheath cells which favours more rapid reductive fixation together with reduced O2 inhibition and lower photorespiration.
C4 plants are partially adapted to drought condition where high rates of CO2 fixation are maintained even with reduced stomatal aperture.
In C4 plants, photosynthesis is more efficient at high temperature whereas the optimum temperature for C3 plants is much lower. Such high-temperature tolerance of C4 plants is due to the stability of some enzymes like PEPcase at high temperature. Oxygen has no inhibitory effect on C4 photosynthesis because PEPcase is insensitive to O2 and photorespiration is absent.
C4 plants are low compensation plants and that means they have the capacity for rapid CO2 uptake even at reduced CO2 levels with almost closed stomata and thus they can conserve water. The C4 syndrome is commonly found in tropical plants which are normally exposed to abundant sunlight which supports higher rates of photosynthesis and growth.
Photorespiration, i.e., CO2 production in light is virtually no detectable from C4 leaves which may either be due to the absence of the enzymes or organelles responsible for photorespiration or to the re-assimilation of photo respired CO2 or to an inhibition of RuBisCO in its oxygenase mode of action by high CO2/O2 ratio developed in bundle sheath cells.
An adequate amount of nitrogen- assimilating enzymes and an efficient capacity to use nitrogen for biomass production are additional features associated with C4.
Another suggested advantage of the operation of PEPcase as the primary carboxylating enzyme is that the C4 plants are able to carry on rapid CO2 fixation under conditions of high stomatal diffusion resistance and consequent large gradient of CO2 between air and leaf interior.
The reason is that PEPcase has higher CO2 affinity as compared to RuBPcase which permits rapid rates of CO2 fixation in spite of low CO2 concentrations in mesophyll caused by high stomatal resistance and consequent limited CO2 diffusion.
Thus, C4 pathway of photosynthesis under hot arid habitats is a device to reduce water loss through transpiration by means of partial stomatal closure so that the plants lose less water for CO2 fixation compared to C3 plants and can make more economic use of water for growth.
Essay # 5. Role of C3 and C4 in Crop Productivity:
Productivity means a total yield. Crop productivity is a multi-factorial phenomenon. It varies widely from almost nothing in some deserts to hundreds of tonnes of dry matter per hectare in tropics and the rates of dry matter production also differ greatly.
For example, crops well-watered and fertilized C3 cereals in temperate zones have the potential to produce more than 25 tonnes of dry matter per hectare in an eight month growing season and in the tropics sugarcane, a C4 plant, forms over 80 tonnes dry matter per hectare per year.
All organic matters is derived from photosynthesis and accumulation of inorganic matter inside the plants requires photosynthetic energy.
Total net photosynthesis per unit ground area is dependent on:
(i) light energy absorbed per unit leaf area
(ii) the response of net photosynthesis to light
(iii) total leaf area
(iv) number of days of assimilation.
Assimilation may be calculated for an entire growing season by integration of photosynthetic rate of individual leaves over light, temperature, CO2 supply, water stress etc., and then over the entire leaf area for each day. The daily assimilation may then be summed over the season to give total assimilation.
(i) Light Intensity:
Light is the driving force for production, and temperature, nutrition and water regulate the production.
Maximum production of photosynthates depends on the photon flux incident upon the canopy and its absorption by leaves in different layers, and on the efficiency of conversion of assimilates. Quantum yield (moles of CO2 fixed per mole of photon absorbed) is the initial slope of the curve relating to CO2 assimilation rate to light intensity.
About 10-15% of incident PAR (photo synthetically active radiation) is reflected and transmitted by leaves, and only a part of the energy not captured by upper leaves in the canopy, is absorbed by other foliage.
As Pn (net rate of photosynthesis) is saturated at photon fluxes of half sunlight, particularly in C3 and shade plants efficiency decreases markedly at intensity above saturation of Pn. Stratification of leaves of different species within a canopy allows very effective light absorption and high productivity.
Crop production is closely related to light interception and, therefore, to leaf area. Efficiency of conversion of light to dry matter in cereals and sugar beet is about 2 g dry matter MJ-1 of PAR absorbed. Conversion of CO2 to crop dry mass depends on the type of organic molecules synthesized and their relative proportion.
One gram of CO2 gives 0.4g fats, 0.62g starch and 0.5g protein, an average value is 0.58g dry matter. Plants producing much oil or protein produce less dry matter than those forming carbohydrates, but the energy yield may be similar.
Each gram of CO2 assimilated is equivalent to an energy content of 38 kJ g-1 in fats, 12.6 kJ g-1 in proteins and 17 kJ g-1 in starch, with an approximate value of 15-20 kJ g-1 in dry matter from the leaves of a range of species.
For a C3 crop requiring 0.5 MJ g-1 dry matter and producing 25 tonnes dry matter per hectare the energy input is 1250 MJ m-2, and the energy content is 50 MJ m-2, and efficiency of about 4 per cent. The conversion efficiency of C4 crops, on the other hand, is 50% greater than C3. They can utilize much higher intensities of light with greater efficiency due to C4 syndrome.
C3 crops are more productive in dim to intermediate light intensities and its canopy architecture influences the efficiency of light utilization.. Many habitats remain dimly lit for long periods with clouds and twilight when the sun’s elevation is low. In that particular condition C3 crops are more productive than C4.
(ii) CO2 Concentration in the Atmosphere:
Oxygen concentration in the canopies of actively photosynthesizing crops is effectively constant. However, carbon dioxide, which is at much lower partial pressure, may decrease substantially within dense, vigorously assimilating vegetation. Internal CO2 partial pressure will then decrease further depending on stomatal conductance.
C3 crops are more affected than C4 because of inefficient rubisco reaction. Stomatal conductance may also decrease due to water shortage, high temperature, etc., and further restrict CO2 supply and decrease photosynthesis; again the effect is relatively greater in C3 plants.
CO2 compensation point in C3 crops varies from 40 – 100µl l-1, whereas in C4 it is 0 – 10µl I-1 due to photorespiration in C3. So, C4 plants are low compensation plants and that means they have the capacity for rapid CO2 uptake even at reduced CO2 levels with almost closed stomata as the primary carboxylating enzyme, PEPcase, has higher CO2 affinity as compared to rubisco.
(iii) Temperature:
Response of Pn to temperature is also very important in determining crop productivity. CO2 assimilation shows an optimum of 20-30°C in typical C3 crops, and 30-40°C in typical C4 crops. Often relatively small differences in temperature have large effects on productivity.
C3 crops may produce more in cool, relatively dimly lit but long growing season than in a warm, bright but short season. Again, this temperature and light dependency in C3 leaves may be altered by changes in the concentration of CO2 or of O2 which increase or suppress photorespiration.
(iv) Water Supply:
C4-plants are partially adapted to drought conditions where high rates of CO2 fixation are maintained even with reduced stomatal aperture. The C4 mechanism brings advantages in water-use efficiency (WUE) to C4 plants. The WUE (g CO2 fixed/kg H2O transpired) in C3 crops ranges from 1 -3g CO2/kg H2O transpired, whereas the value ranges from 2-5 in C4 crops.
The fact that C4 photosynthesis can operate at low intercellular concentrations of CO2, and hence lower stomatal conductance, means that C4 plants can restrict water loss to a minimum. Thus, WUE in C4 plants is roughly double than that of C3 plants.
(v) Nutrition:
Nutrition is an important factor for crop productivity. So, to get maximum yield proper and balanced fertilizer practice of the soil is necessary.
(vi) Physiological State of the Plant:
Physiological state means the plant and leaf age and reproductive state of the plants, on which productivity depends largely along with other factors. Nutrition and physiological state interact irrespective of the C3 or C4 type of the crop.
Photosynthetic rate is a major determinant of dry matter production in both types of crops. C4 and C3 crops with average Pn in bright light of 30 and 13µ mol CO2 m-2 leaf s-1 respectively, have growth rates of 22 and 12g m-2 ground day-1. Yield of C4 crops is correspondingly greater than C3.
The greater productivity of C4 crops is largely either due to lack of photorespiration or due to absence of enzymes or organelles responsible for photorespiration or to the re-association of photo respired CO2 or to an inhibition of rubisco in its oxygenase mode of action by high CO2/O2 ratio developed in bundle sheath cells.
Again, an adequate amount of nitrogen-assimilating enzymes and an efficient capacity to use nitrogen for biomass production are additional features associated with C4. Average yield in U.S.A. of C3 plants of wheat and soybean are 2.5 tonnes/hectare and of rice 5.8 tonnes/hectare, while that of the C4 species maize is 7.8 tonnes/hectare.
Essay # 6. Role of Light in Photosynthesis Control (Metabolic Regulation):
(i) Light Activation of Photosynthesis:
Light drives photosynthesis by activating several Calvin cycle enzymes via thioredoxin and changes in stromal pH and magnesium. When isolated chloroplasts or leaves are illuminated, it leads to alkalization and increased free Mg2+ in stroma. Several enzymes of the Calvin cycle including pFBPase (plastidial FBPase), SBPase and Rubisco are strongly activated by increasing pH and Mg2 +.
The pH and magnesium sensitivity of pFBPase is due to an increase in the proportion of the total FBP pool present as FBP4-Mg2 +, which is the real substrate. An analogous explanation is possible for SBPase, pFBPase, phosphoribulokinase (PRK) and NADP + – glyceraldehyde phosphate dehydrogenase in Calvin cycle.
An enzyme termed ferredoxin-thioredoxin oxidoreductase transfers reducing equivalents from ferredoxin to a small soluble protein called thioredoxin. Thioredoxin then modifies the cysteine groups constituting the active sites on the target protein.
Further, enzymes outside the Calvin cycle including NADP+ -malate dehydrogenase and CF1 – ATP synthase in thylakoids are also activated by thioredoxin. The precise effect of thioredoxin activation of these enzymes is an increased substrate affinity.
(ii) Light Activation of Rubisco:
Rubisco is unique, as the catalytic site of the active enzyme contains a carbamylated lysine to which Mg2 + is complexed (termed ECM form). The enzyme can be carbamylated or de-carbamylated in vivo, leading to change in ‘activation state’ [ECM/(E + ECM)]). Activation state increases as irradiance increases. Hence rubisco activation is dependent on light. A protein called Rubisco Activase is needed to enable these changes.
Activase operates by decreasing the affinity of de-carbamylated enzyme for various sugar bisphosphates, which would otherwise inhibit carbamylation of Rubisco or catalysis. Release of the bound sugar bisphosphates requires ATP hydrolysis.
Inhibitory sugar bisphosphates include RuBP itself, which binds tightly to the de-carbamylated form of Rubisco. Rubisco activation is regulated to achieve a balance between RuBP formation and its consumption, such that the active rubisco sites (ECM) are almost fully saturated by RuBP.
An unusual inhibitory sugar phosphate, carboxyarabinitol-1 –phosphate (CAP), is a closely related structure to 2-carboxy-3-keto-arabinitol 1, 5-bisphosphate, which is the transition state analog of carboxylation reaction (i.e., 2-carboxy-3-ketoribitol 1,5-bisphosphate). CAP binds with high affinity to the ECM form (i.e., active form) of Rubisco and depresses Vmax.
Leaves contain considerable amount of free sugar carboxyarabinitol (CA) in light and dark. CA can act as a precursor of CAP in dark and CAP is converted back to CA after illumination. This is another aspect of light regulation of Rubisco.
(iii) Photo Inhibition of Photosynthesis:
Photo inhibition is a physiological stress condition induced in plants as a consequence of an imbalance between the number of photons captured and their utilization in photosynthesis. Photosynthetic activity is depressed by excess light affecting both plant growth and productivity.
The most vulnerable part of photosynthetic apparatus is photosystem II (PSII) which powers water oxidation and oxygen evolution. During light-induced damage of PSII, a key component of its reaction center, i.e., D1 turns over far more rapidly than any other protein in the photosynthetic membrane.
This turnover is a part of a repair system that functions to replace damaged reaction center with newly synthesized D1 protein and thus restore normal PSII activity.
If the rate of repair of PSII does not keep pace with its rate of damage, then photo inhibition is observed in the form of decrease in photosynthetic activity. When the repair system copes with the rate of damage, then no loss of photosynthetic capacity is observed.
Photo inhibition of photosynthesis is often enhanced by other stress conditions, such as nutrient and water shortage and abnormally high or low temperatures. It has been observed that the turnover of D1 protein not only occurs at excess light intensities, but also at non-saturating light.
The conclusion is that the photo inhibition of PSII is an intrinsic phenomenon, which occurs over a wide range of light intensities. Therefore, even at moderate light intensities, where no net reduction of photosynthetic capacity can be seen, plants are still suffering from light induced stress.
The reason is that the maintenance of a repair system (i.e., protein synthesis) is always an energy consuming phenomenon and there will be a distinct depletion of metabolic reserves to achieve this.
PSII performs the unique chemistry of water splitting. The chemical reactions involved in this process are dangerous for the protein environment in which they occur for two reasons. In the first place, sufficiently high oxidizing potentials are required to extract electrons from water by its oxidation, so it naturally follows that other detrimental oxidation reactions are possible.
Secondly, oxidation of water results in the formation of molecular oxygen as a by-product. This molecular oxygen can readily form highly reactive oxygen species, which can attack proteins and other PSII components.
PSII acts as a water-plastoquinone reductase system which has a 4-electron gate on its oxidizing side and a 2-electron gate on its reducing side. Although PSII is a multi-peptide complex with more than 20 subunits, these redox reactions are restricted to only two polypeptides. D1 and D2 proteins and these proteins form the reaction center of PSII.
It is likely that the primary electron donor P680 is composed of two chlorophyll molecules that are ligated to and adpressed between D1 and D2 proteins. Active Phe is bound to D1 protein and an inactive Phe is located by two-fold symmetry on the protein. Plastoquinone acceptor QA is on D2 and QB on D1 protein.
A non-heme iron is placed equidistant between QA and QB.
When fully reduced and protonated, QB plastoquinone (PQbH2) diffuses out of QB binding site and is replaced by a fully oxidized plastoquinone molecule available from plastoquinone pool in the lipid matrix of thylakoid membrane. In this way, reducing equivalents are removed from PSII reaction center and made available to subsequent electron transfer processes to be taken over by PSI.
On the oxidizing side, the tyrosine residue (Yz) that acts as an intermediate between manganese cluster and P680 is lying on D1 protein. The manganese cluster is probably ligated to surfaces of D1 and D2 proteins.
No detailed information is available for the structure of PSII reaction center of higher plants, but molecular models have been constructed based on analogy with its counterpart in purple bacteria where the structure has been determined by X-ray crystallography.
(iv) Photochemical Processes Giving Rise to Damage:
Initial damage to PSII involves photochemical processes that inactivate its function and trigger D1 and D2 proteins for enzymatic degradation by protease. Two distinct pathways by which photo damage may occur, one induced from the acceptor side of PSII, and another from the donor side of PSII.
(a) Acceptor Side-Induced Photo-Damage:
The idea that photo inhibition involves the acceptor side of PSII arises from the fact that there is a link between stress and turn-over of D1 protein. It was postulated that photo inhibition was due to the interaction of molecular oxygen with plastoquinone in QB side and the resulting oxygen radicals would in turn lead to protein damage, thereby inhibiting electron transport.
It has been shown that exposure of isolated thylakoids to photo inhibitory light is accompanied by D1 protein degradation and singlet oxygen (1O2) production. Singlet oxygen is responsible for protein damage. Besides singlet oxygen, other toxic oxygen like superoxide and hydroxyl radicals may also be involved in damage.
(b) Donor Side-Induced Photo-Damage:
A second route by which PSII can be photo inactivated has been suggested which is oxygen-independent. This photo inactivation occurs when the rate of electron donation to PSII does not match the rate of electron removal to the acceptor side. This will destabilize the water splitting system in the donor side and such destabilization will result in an increased lifetime for P680+ state.
Since P680+ has a high oxidizing potential, this has the capacity to extract electrons from its surroundings and thus cause oxidative damage to the protein environment with pigments like carotenoids and chlorophylls.
The site of donor side inactivation is between manganese cluster and P680 and electron transport will be impaired owing to oxidative damage by P680 +. In addition to irreversible bleaching of pigments, detrimental oxidation of amino acids is also possible.
Both acceptor and donor side mechanisms of photo inhibition involves oxidative damage. Such oxidative damage may induce a conformational change in the reaction center, which can serve as a sensing signal for proteolytic cleavage. This conformational change is essential for turning D1 protein into a substrate for proteolysis.
Essay # 7. Effects of Changing Temperature and Atmospheric Carbon Dioxide Concentration on Photosynthesis:
When the terrestrial plants first appeared in the ancient geologic time, the atmospheric CO2 concentration was several fold higher than today. It was seen to fluctuate in the past, recording both rise and decline. Ultimately, the CO2 concentration became reduced to low levels near the present values.
It is believed that the relative abundance of land plants was mainly responsible for the substantial reduction in the CO2 concentration by means of photosynthetic assimilation. The enzyme ribulose 1, 5-bisphosphate carboxylase-oxygenase (Rubisco) is the entry point for inorganic carbon into the photosynthetic carbon reduction (PCR) cycle.
Rubisco regulates CO2 assimilation as the primary CO2-fixer in C3 plants which constitute 95% of the plant species. Atmospheric O2 also interacts with rubisco as a competitive inhibitor with respect to CO2 and as a substrate for this bi-functional enzyme to produce phosphoglycolate, which is partially metabolized to CO2 in the photo respiratory carbon oxidation cycle.
Consequently, Rubisco is not saturated by CO2 in the present gaseous composition of the atmosphere. Thus the present-day plants have developed several photosynthetic adaptations which facilitate survival in a CO2-depleted atmosphere. These adaptations include greater specificity of rubisco for CO2 which permits its carboxylase mode of action and the operation of a CO2 concentrating mechanism (CCM).
In C3 species, O2 produces detrimental effects in favouring photorespiration. The C4 and CAM pathways may be regarded as a common photosynthetic modification with a CCM developed in response to declining CO2 of the atmosphere. The plants have further developed pathways to recapture carbon and nitrogen lost in photorespiration.
By means of C2 pathway of photosynthesis, C3 plants can conserve 75% of the carbon that enters into phosphoglycolate. A further efficient system can be afforded by C3-C4 intermediate plants, in which the CO2 representing the remaining 25% of the carbon released in photorespiration by glycine decarboxylase present in bundle sheath cells will be re-fixed by Rubisco before it can escape.
For terrestrial plants with an abundant water supply, another consequence of the decrease in CO2 is seen in stomata remaining fully open which compensate for the lower CO2 level. However, when water is limited, rapid stomatal response and improved stomatal efficiency are adopted to ensure maximum CO2 gain in terms of water lost.
Under the present atmospheric CO2 concentration, i.e., around 300-350 ppm, rubisco is not saturated. However, the potential for CO2 enrichment to stimulate photosynthesis and growth has been recognized for a long time. When the atmospheric CO2 level is approximately doubled, the net photosynthesis of the major C3 crop species initially increased appreciably, but declined after prolonged exposure.
Despite the lowering trend of photosynthetic rate under long-term exposure to doubling of CO2, changes in parameters like leaf area and duration can result in greater biomass and yield in plants growing in CO2-elevated atmosphere.
The underlying causes of the improved biomass and yield under CO2 enrichment are inhibition of photorespiration by reducing the O2 inhibition of rubisco together with lowering of dark respiration, and enhancement of water use efficiency (WUE) by reducing stomatal conductance.
It is, however, necessary to realize that environmental conditions often influence the degree to which a rising CO2 regime can stimulate photosynthesis and growth.
Effect of Changing Temperature on Photosynthesis:
Photosynthesis is an integration of photochemical as well as biochemical processes. Thus, temperature fluctuations will have a direct impact on photosynthesis through its effects on the temperature-sensitive biochemical and physiological processes.
These include:
(i) Photosynthetic carbon reduction
(ii) Sucrose and starch synthesis
(iii) Carbon partitioning between source and sink organs
(iv) Electron transport associated with photosystems.
The combined effect of light and temperature can lead to metabolic imbalance which, in turn, can result in a significant decrease of photosynthesis as a result of photo inhibition. With regard to the daily and seasonal fluctuations in temperature, the responses in plants may be separated into two principal components.
First, adaptation is a genotypic response and the alterations caused by temperature changes are stable which will remain in the population over generations.
Second, acclimation is a response induced by an environmental change which causes a phenotypic alteration over a single generation without any compositional change in the genetic complement.
There is no general mechanism for photosynthetic acclimation to high or low temperature. This is because high temperature and low temperature are relative terms. This means that low temperature for one plant species may become high temperature for another species.
At low temperature (0 – 10°C), photosynthesis is limited thermodynamically. Sub-lethal low temperatures can directly exert a reversible limitation on photosynthetic rate caused by thermodynamic restriction on enzyme-catalyzed reactions. This may be manifested by phosphate limitation in chloroplasts mainly due to reduced rates of sucrose synthesis.
In contrast, supra-optimal high temperature (35 – 50°C) cause irreversible inhibition of CO2 uptake or O2 evolution primarily due to destabilization of thylakoid membrane. In contrast to low temperature, light can protect against high temperature inhibition of photosynthesis.
A direct relationship has been noticed in cyanobacteria between increased unsaturation of membrane lipid and adjustment to low temperature. However, the evidence for this correlation is lacking in algae and higher plants. Temperature may also influence photosynthetic capacity and efficiency indirectly through inhibition on chloroplast development.
It appears that under low temperature regime, the formation of chloroplast gene products is more sensitive to temperature change than the formation of nuclear gene products. This may be the reason for the sensitivity of chloroplast development to low temperature.
Essay # 8. Photosynthetic Carbon Oxidation Cycle:
The most common mechanism for the reduction of CO2 to carbohydrate has been elucidated by Calvin and his associates.
It has been referred to as the C3 photosynthetic carbon reduction cycle or in short, the C3 cycle. Intimately connected with and dependent on the C3 cycle, there is a mechanism for the oxidation of carbohydrates to CO2 and this light-mediated CO2 evolution is absolutely different from the well-known normal dark respiratory process.
The close connection between carbon reduction cycle and carbon oxidation cycle is established by the enzyme ribulose-1, 5-bisphosphate carboxylase (RuBPcase) initiating the cycle, which has the remarkable property of fixing not only CO2 but also O2 (Bowes et al., 1971; Andrews et al., 1973; Lorimer et al., 1973).
Hence the name of the same enzyme performing dual roles, is RuBP carboxylase oxygenase or RuBisCO in short.
The pathway involving the fixation of O2 as the primary event followed by further O2 uptake and CO2 evolution in light has been referred to as the C2 photosynthetic carbon oxidation cycle or C2 cycle, named after the C2 compound glycolate occurring in the metabolic sequence. The integration of the C3 carbon reduction cycle with the C2 carbon oxidation cycle is shown in Fig. 8.32.
Essay # 9. Biochemistry of C2 Cycle:
(i) Formation of Glycolate and Its Oxidation:
The present formulation of the glycolate pathway or C2 cycle of photosynthesis has been reviewed by Chollet and Ogren (1975), Tolbert (1980), Lorimer and Andrews (1981) and Bidwell (1983). The scheme shown in Fig. 8.18 coordinates several components of the C2 cycle and its complementary metabolic pathways.
The C2 cycle starts with the production of phosphoglycolate in chloroplasts by RuBP oxygenase action, the other product being a molecule of PGA. The enzymes responsible for catalyzing the various reactions of C2 cycle are not confined to one cellular compartment, instead its operation involves the cooperation of three organelles, viz., chloroplasts, peroxisomes and the mitochondria.
The phosphoglycolate undergoes de-phosphorylation by phosphoglycolate phosphatase and the glycolate (a C2 compound) so formed leaves the chloroplast and enters the peroxisome where it is oxidized to glyoxylate by glycolate oxidase. The other product of glycolate oxidation is H2O2 which is decomposed to H2O and O2 by catalase, a constituent of peroxisomes.
It is possible that some of the glyoxylate is returned to the chloroplast where it may be reduced back to glycolate at the expense of photo generated NADPH by glyoxylate reductase. Such shuttle reactions involving glycolate- glyoxylate are used to dissipate the light-generated reducing power for preventing damage to the photosynthetic apparatus, when CO2 supply is limited.
Photorespiration is observed in higher plants which photo synthetically fix CO2 exclusively via the Calvin cycle. Glycolate is an early product of C3 photosynthesis and this C3 acid has been implicated as an intermediate in photorespiration.
Glycolate synthesis and photorespiration are stimulated by O2 and depressed by CO2, whereas photosynthesis is stimulated by CO2 and inhibited by O2. This competition between CO2 and O2, like CO2 promoting photosynthesis and inhibiting glycolate synthesis and O2 promoting glycolate synthesis and inhibiting photosynthesis, has been termed the Warburg effect.
There is some controversy regarding the source of glycolate and different pathways have been proposed for its formation.
Tolbert’s group, as early as 1973, offered convincing evidence to prove that glycolate is produced from RuBP by the RuBP oxygenase reaction. An alternative pathway for glycolate synthesis was proposed by Zelitch (1965) who found that the radioactivity of photosynthetic glycolate in tobacco leaves exposed to 14CO2 was higher than that of PGA or P-glycolate.
This observation led him to conclude that glycolate was synthesized by direct condensation of two molecules of CO2 in light in a reaction sequence bypassing the Calvin cycle. However, no enzymatic confirmation has been made and thus Zelitch’s proposal is considered largely speculative.
A second alternative has been suggested by Shain and Gibbs (1971).
According to them an enzyme of the Calvin cycle, transketolase, catalyzes the transfer of 2C fragment (dihydroxyethylthiamine pyrophosphate or active glycol aldehyde) from certain sugar phosphates which can be oxidized non-enzymatically to glycolate and TPP either by a PS II oxidant or by H2O2 generated by auto-oxidation of reduced ferredoxin formed by the light reactions of photosynthesis.
According to this scheme, the Warburg effect may result from a competition between CO2 and O2 for photo synthetically-derived reductant ferredoxin. In low CO2 and high O2, little PGA will be available for reduction to triose phosphate, so the reduced ferredoxin is oxidized by O2 with H2O2 production, which then oxidizes dihydroxyethylthiamine pyrophosphate to glycolate.
In high CO2 and low O2, much PGA is available for reduction, so the reduced ferredoxin reduces NADP+ and the resultant NADPH reduces 3PGA to glyceraldehyde-3-phosphate.
Thus, low CO2 and high O2 stimulate peroxide formation and therefore photorespiration while high CO2 and low O2 stimulate PGA reduction and therefore photosynthesis. But recent evidence shows that H2O2 inhibits rather than stimulates glycolate synthesis.
Another objection is that this pathway produces glycolate whereas phosphoglycolate, the product of RuBP oxygenase, is considered to be the precursor of glycolic acid. Finally, about 50 per cent of the total CO2 fixed in photosynthesis is believed to flow through glycolate.
Such a massive flow of carbon through a non-enzymatic pathway is not possible in biology. Most recent experimental evidence, however, supports that RuBP oxygenase reaction is the major source of photosynthetic production of glycolate.
(ii) Fate of Glycine:
Glyoxylate is aminated to glycine by peroxisomal transaminase, glutamate-glyoxylate aminotransferase. Then glycine, in turn, passes from peroxisome to the mitochondrion. Here two molecules of glycine are converted to one molecule each of serine, CO2 and NH3 in a two-step process. This conversion of glycine to serine in mitochondria is the source of CO2 evolution in photorespiration.
In the first step, one molecule of glycine is degraded by the enzyme glycine decarboxylase into three components. The carboxyl and amino groups are eliminated as CO2 and NH3 respectively, while methylene carbon becomes bound to tetrahydrofolate (THF) as N5.10-methylene THF capable of one-carbon transfer reaction.
This reaction requires NAD+ as an oxidant and the resultant NADH is re-oxidized by mitochondrial electron transport chain with the generation of ATP (Keys etal., 1978; Sawhney et al., 1978). However, Woo (1980) points out that the electron transport chain may not be functional in light, instead NADH is used to reduce oxaloacetic acid produced in the peroxisomal reduction of hydroxypyruvate to glycerate.
In the second step, both methylene carbon of N5, 10-methylene THF and a second molecule of glycine are involved in the formation of serine catalyzed by serine hydroxymethyltransferase. The photo respiratory nitrogen metabolism is shown in Fig. 8.33.
Ammonia so produced in glycine decarboxylase reaction is recycled during photosynthesis which is necessary to conserve nitrogen within the plant and to prevent toxic ammonia to accumulate within the mitochondria. Ammonia produced in mitochondria moves out in the cytoplasm and is accepted by glutamate in the presence of glutamine synthetase (GS).
Glutamine so formed passes into the chloroplast where two molecules of glutamate are formed by the action of glutamine:
Oxoglutarate aminotransferase (GOGAT).
Of the two glutamate molecules produced in the chloroplast, one molecule is used to continue the GS reaction for glutamine synthesis while the second molecule enters the peroxisome to donate its amino group to glyoxylate to form glycine in the transaminase reaction. In the last reaction (GOGAT), the energy-requiring step is coupled to the energy-releasing process of re-oxidation of reduced ferredoxin.
Thus, nitrogen cycles of photorespiration are important complementary metabolic pathways of photosynthesis. Alternative fates of amino acids glycine and serine of C2 cycle are possible. For instance, some glycine and serine may be used up in protein synthesis and glycine may also be a substrate of chlorophyll synthesis.
(iii) Glycolate Pathway to Sugars:
Serine, once formed in the mitochondria, can be further metabolized in peroxisomes to hydroxypyruvate by serine-glyoxylate aminotransferase. The hydroxypyruvate, in turn, is reduced to glycerate by peroxisomal NADH-hydroxypyruvate reductase.
NAD thus produced in peroxisomes is coupled to the oxidation of malate to oxaloacetate which is transferred to-mitochondrion where it is reduced back to malate by the reducing power (NADH) generated in the hydroxymethyltransferase reaction.
Malate would again shuttle back to peroxisomes for the reduction of hydroxypyruvate. Glycerate produced by hydroxypyruvate reduction can be reduced to triose phosphate in the cytoplasm and may be used as a substrate for sucrose synthesis directly without passing through the C3 cycle.
It is, however, more probable that glycerate enters the chloroplast and is phosphorylated to phosphoglycerate (PGA) by glycerate kinase which enters the Calvin cycle and is finally converted to carbohydrate.
This cycle may be used for scavenging purpose that recovers 75 per cent of the carbon lost as glycolate back into PGA and 25 per cent is liberated as photo respired CO2. In addition, the various intermediates like glycine, serine and glycerate formed in the C2 cycle may meet the demand for cellular synthesis.
From Fig. 8.33, the balance of O2 uptake and CO2 liberation may be obtained. In each turn of the C2 cycle, two molecules of O2 are utilized for the oxidation of two molecules of glycolate whereas one molecule is produced by the catalase reaction, thus keeping one molecule of O2 net absorbed.
Oxygen absorbed by the oxygenase reaction is balanced equally by O2 produced in the light reaction. Since one molecule of CO2 is produced in each turn of the cycle, the balance is one molecule of O2 absorbed per molecule of CO2 produced and the RQ(CO2/O2) will be 1.0.
Zelitch (1972) criticized the chloroplast-peroxisome-mitochondrion pathway of photo respiratory glycolate metabolism because the conversion of glycine to serine is not so rapid as to account for the observed photo respiratory rates. He proposed that glyoxylate rather than glycine undergoes oxidative decarboxylation to produce the photo respiratory CO2 in chloroplasts.
Zelitch suggested that glyoxylate, product of glycolate oxidation in peroxisomes is transported back to chloroplasts and non-enzymatically oxidized there to CO2 and formate by the H2O2 formed during the light reactions of photosynthesis.
The resulting formate (IC compound) would form a tetrahydrofolate derivative-which takes part in the serine hydroxymethyltransferase reaction in the mitochondria. It, however, seems likely that glyoxylate oxidation is not a regular feature, it may be a source of photo respiratory CO2 under stress conditions.
Essay # 10. Bacterial Photosynthesis:
Bacteria are usually heterotrophic organisms living as parasites or saprophytes There are however, some bacteria which are able to synthesize organic matter from carbon dioxide in light.
These photosynthetic bacteria are pigmented, live in media containing sulphide and are strictly anaerobic. They cannot use water as the source of electrons for reducing carbon dioxide and they do not evolve oxygen during photosynthesis.
There are three major groups of photosynthetic bacteria.
These are:
1. Green sulphur bacteria (Chlorobiaceae and Chloroflexaceae) represented by Chlorobium and Chloroflexus. They can use sulphide or thiosulphate as electron source.
2. Purple sulphur bacteria (Chromatiaceae) represented by Chromatium which can grow by utilizing hydrogen sulphide as electron donor.
3. Purple non-sulphur bacteria (Rhodospirillaceae) represented by Rhodospirillum, Rhodopseudomonas and Rhodomicrobium. They cannot use hydrogen sulphide and can utilize simple organic compounds like alcohols and acids as electron donors.
In green plant photosynthesis, chlorophyll a together with α- and β-carotenes are the common pigments having major absorption bands in the visible range of the light spectrum. The chlorophyllous pigments of bacteria are called bacteriochlorophylls which, however, absorb near-infrared radiation.
This confers on the photosynthetic bacteria the ability to grow in places where higher green plants will not survive. Bacteriochlorophyll is the main pigment of purple sulphur and non-sulphur bacteria. The absorption maxima in vivo are situated between 800 and 900 nm, whereas extracts of bacteriochlorophyll in organic solvents show a major absorption peak at a much shorter wave length, 770 nm.
It has been suggested that there may be a BChl a and BChl b. Green bacteria contain mostly the so-called Chlorobium chlorophyll or bacteriovirdin. Two types of Chlorobium chlorophyll have been identified.
The absorption maximum in vivo of these pigments is about 750 nm. The absorption is shifted to lower wavelengths in extracts of the pigments in organic solvents and thus two types of Chlorobium chlorophyll like Chlorobium chlorophyll 660 and Chlorobium chlorophyll 650 have been shown to exist.
In addition, a chlorophyll spectroscopically identical to BChl (i.e., the main pigment of purple bacteria) is also present in Chlorobium. The pigment in vitro absorbs strongly in ether solution at 770 nm and is named Chlorobium chlorophyll 770. The absorption spectra of two typical bacteria are shown in Fig. 8.36.
Bicyclic carotenes (α- and β-carotenes), common in algae and higher plants are rare among the photosynthetic bacteria. Most of the carotenoids of photosynthetic bacteria are open-chain aliphatic molecules and lycopene is of wide occurrence in bacteria. One monocyclic carotenoid, γ-carotene has been identified in green bacteria.
The bacteria do not have organelles like typical chloroplasts. However, the pigments of the photosynthetic apparatus remain bound to protein and consist of physically homogeneous particles.
These pigment-protein particles can be isolated by differential centrifugation and are designated as chromatophores which are the photosynthetic organelles in prokaryotic organisms. Each chromatophore contains several photosynthetic units.
The analysis of chromatophores isolated from Rhodospirillum sphaeroides show that these consist of 40 reaction center complexes, 500 light- harvesting complexes (LH), 1000 carotenoids and 1000 ubiquinone molecules. Cells of photosynthetic bacteria utilize the energy of light to oxidize inorganic sulphur or organic compounds and have the ability to couple this with the reduction of CO2.
A probable sequence of electron transfer catalyzed by chromatophores from bacteria is shown in Fig. 8.20. The scheme indicates that light is absorbed by bacteriochlorophyll from which an electron is ejected which travels to ferredoxin. Ferredoxin, in its turn, donates electron to NAD and the NAD photo reduction occurs via a flavoprotein. Both ferredoxin and flavoprotein (FMN) may channel electrons to ubiquinone.
Organic substrates like succinate or fumarate may donate electrons to ubiquinone or ubiquinone may reduce organic substrate or transfer electrons to RHP (Rhodospirillum Heme Protein) which is unique in bacterial photosynthesis. Cytochromes b and c2 (cf) are positioned after RHP in the electron transfer sequence.
Photophosphorylation is a common feature of photosynthesis by both bacteria and higher plants. While photosynthetic phosphorylation in chloroplasts was discovered by Arnon et al., (1945), photophosphorylation in bacterial chromatophores was discovered by Frenkel (1954).
In bacterial photosynthesis, cyclic photophosphorylation is the dominant photochemical process. Since photosynthetic bacteria do not evolve oxygen and oxygen evolution takes place during non-cyclic photophosphorylation, it was previously thought that the non-cyclic process is absent in bacteria.
Later it was demonstrated that a non-cyclic process without O2 evolution also occurs in bacteria which is dependent on the unidirectional flow of electrons from some donor, viz., thiosulphate, H2S, organic acids, DPIP-ascorbate to NAD acting as an electron acceptor (Losada ef al., 1961; Nozakief al., 1961).
Thus, the photosynthetic bacteria can generate both ATP as energy and NADPH as reducing power for the fixation of CO2. Both cyclic and non-cyclic pathways of electron transfer are outlined in Fig. 8.38.
Since the labeled products of bacterial photosynthesis with radioactive 14CO2 are similar to the products of green plant photosynthesis, it is presumed that CO2 fixation in bacterial photosynthesis takes place via the Calvin cycle.
A totally new cycle for CO2 fixation has been described by Arnon and his associates (Evans et al., 1966) in Chlorobium and Chromatium in which the strong reducing power of ferredoxin causes the reversal of two reactions of the tricarboxylic acid cycle (TCA cycle). These are pyruvate synthetase and α-ketoglutarate synthetase reactions, in which CO2 fixation takes place through the synthesis of α-keto acids.
They call the cycle the reductive carboxylic acid cycle (Fig. 8.39).
In each turn of the cycle four molecules of CO2 are fixed leading to the net synthesis of one molecule of oxaloacetic acid. This oxaloacetic acid may undergo further metabolism to provide different carbon compounds for the synthesis of amino acids, lipids and other cellular constituents.
A Comparison of Plant and Bacterial Photosynthesis:
While comparing plant and bacterial photosynthesis, let us first consider the similarities. We find that bacteriochlorophyll a and chlorophyll a are more or less identical, bacteriochlorophyll a, however, contains two more H atoms (in ring B) than chlorophyll. A reaction center is present in both systems and the accessory pigments also serve similar functions. Both types of chlorophyll remain associated with membranes.
In both green cells and bacteria, the overall process of photosynthesis is an oxidation-reduction reaction. A reductant H2A is used to reduce CO2 in the presence of chemical energy generated in light leading to the release of an oxidized product A. Both cyclic and non-cyclic photophosphorylation processes exist in green plants and bacteria.
The components of the electron transport chain appear to be almost similar in green plants and photosynthetic bacteria. In both organisms, chemical energy and reductant generated in light are used in carbon reduction. In green plants, the photo generated reductant is NADPH, whereas it is NADH in bacteria.
The major distinction between plant and bacterial photosynthesis is that the bacteria have no distinct chloroplast-like structure but they possess membrane-like structures of particles called chromatophores.
In green sulphur bacteria, however, non-membranous particulate structures, called chromosomes which have been demonstrated in LH (light-harvesting antenna pigments) and RC (reaction centre) complexes are located.
In purple bacteria, these are present within a membrane system. Another distinguishing feature is that bacteria do not evolve oxygen. Therefore, they do not use water as the source of electrons, on the other hand, they use H2S and other reduced compounds as the source of reducing power.
Thus, those organisms (plants, algae and Cyanobacteria) that utilize water are said to carry on oxygenic or plant photosynthesis. The organisms (purple, and green bacteria) that utilize other electron sources are said to carry on an oxygenic or bacterial photosynthesis.