The following points highlight the seven main types of photoreceptors of photosynthetic pigments. The photoreceptors are: 1. Phycobilins 2. Cryptochrome 3. UV-B Receptors 4. Flavonoids 5. Betacyanins 6. Chloroplasts 7. Carotenoid Pigments.
Photoreceptor # 1. Phycobilins:
These are present in red algae, cyanobacteria or even green plants and serve as accessory light- harvesting pigments.
Four phycobilins are reported; three are involved in photosynthesis and the fourth regulates various aspects of growth and development. It is called phytochromobilin.
Phycoerythin, phycocyanin and allophycocyanin and involved in photosynthesis. Phycobilin pigments have open chain tetrapyrrole and a protein as its integral part, a chromoprotein. The complete molecule (holochrome) consists of the chromophonre plus the apoprotein. Phycobilin proteins are organized into phycobilisomes.
Only phytochromobilin is found in higher plants. Phytochrome plays an important role in many photomorphogenic phenomena and exists in two forms that are photo reversible (Pn and Pfn). Pfn is an active form of the pigment.
Phycocyanin and phycoerythrin are two other photosynthetic pigments present in blue green and red algae. The former is blue, while the latter is red. They are commonly known as phycobilins and are also tetrapyrroles where the four pyrrole rings are phytol.
They are attached to proteins and are water soluble. Both absorb light and cause photosynthesis. In the red algae, the highest efficiency is in the green region (495 to 595 nm) where absorption by phycoerythrin is maximum and by the chlorophyll the least.
The absorption spectrum phycocyanin has peaks at 500 and 615 nm. The role of phycobilins is indirect. They absorb light energy and transfer it to chlorophyll a which utilises it to affect photosynthesis. Such pigments and also carotenoids are called accessory pigments. In addition chlorophylls b, c and d are also considered accessory pigments.
Photoreceptor # 2. Cryptochrome:
Several responses to blue and UV-A radiation have been observed for a long time but the precise identity of their photoreceptor was not unravelled—thus the name cryptochrome. Carotenoids and flavins, or both, were suggested as possible contenders since they were omnipresent in living organisms.
Three most common flavinsare, riboflavin, FAD and FMN and occur free or complexed with proteins (flavoproteins). FAD and FMN, are important cofactors in oxidation-reduction reactions within a cell. Obviously flavoproteins constitute a very small portion of the pool and hence very tedious to isolate and characterize.
Recent studies combining genetic, photobiological and biochemical approaches in Arabidopsis, HY4 gene has been identified to encode the blue photoreceptor that mediates inhibition of hypocotyl elongation. The product of this gene is CRYL-a cytoplasmic protein, similar to photolyase (flavoproteins that use blue light to enhance UV-induced damage to microbial DNAs. The general view is that CRYl could be cryptochrome.
Photoreceptor # 3. UV-B Receptors:
Recent findings in young Sorghum vulgare and carrot cells suspensions have indicated the presence of one or more UV-B receptors in plants though the exact nature of the photoreceptors is still evasive. The UV-B receptor seems to modulate responses to phytochrome in some systems.
Photoreceptor # 4. Flavonoids:
Various shades of scarlet, purple, pink and blue in flowers is attributed to anthocyanins and the latter belong to flavonoids. Some flavonoids like chalcoes and chalcones are responsible for yellow colour of some flowers. White floral petals are due to flavones.
Flavonoids are phenylpropane derivatives with a basic C6 – C3 – C6 composition and the basic skeleton for the group is flavan. In all there are twelve groups of flavonoids and three constitute major ones and these are flavones, flavonols, and anthocyanadins. Anthocyanidins and anthocyanins are most strongly coloured and the former are of twelve types.
The anthocyanins are water soluble and are found in the vacuolar sap and can be extracted in acidic solution. In leaves of some plants like Coleus, Acer anthocyanins are found in the vacuoles of the epidermal cells and appear to mask the chlorophylls.
In view of their different absorption spectra their presence does not interfere with photosynthesis carried out by the chloroplasts present in the mesophyll cells situated below. One possible function of flavonoids is to protect the underlying leaf tissue from damage due to UV radiation.
Isoflavonoids are shown to exhibit antimicrobial activity and some act as phytoalexins. The synthesis of phytoalexins in response to fungal attack or some elicitors limits the spread of the pathogen.
Photoreceptor # 5. Betacyanins:
Red pigments in beet root and Bougainvillea bracts are complex group of glycosylated compounds called betacyanins or betalains. These pigments do not respond reversibly to changed pH and the molecule contains nitrogen.
Photoreceptor # 6. Chloroplasts:
Electron microscopy has revealed that eukaryotic chloroplast is surrounded by an outer differentially permeable wall consisting of two smooth membranes having no perforation and inside it has a proteinaceous matrix, the stroma.
The inner membrane extends at several places across the plastid to form a lamellar system. At various points, these extensions unite with an aggregate of nearly identical membranes. Each such stack is like a pile of coins and is called a granum.
Usually 40 to 60 grana are present in a mature chloroplast. These membranes are paired. In the region of stroma they are thin and are called stroma lamellae. The photosynthesis pigments are confined to the lamellar system of the chloroplast.
The flattened membrane sac in chloroplast is called thylakoid while the coin-shaped component of a granum is grana thylakoid; Stroma thylakoids connect the adajcent grana. Thylakoids are also known as lamellae. The space within stroma thylakoids are continuous with thylakoid spaces of some individual grana thylakoid.
In a granum there may be as many as one hundred stacked thylakoids. There are also darkly- staining round granules (plastoglobuli), several ribosomes and aggregation of DNA fibrils. Plastoglobuli are made up of vitamin K, plastoquiones, etc. They are thought to play some role in membrane biosynthesis.
Mitochondria and chloroplast have several features in common. For instance, presence of more than one membrane envelope, having DNA and ribosomal machinery and lipoprotein membranes having ADP phosphorylating system, etc. But they differ in function, number and shape in a given cell, besides organization.
In blue-green algae thylakoids are distributed in the cytoplasm with regular spacing and are never enclosed within definite organelles. Thylakoids are situated peripherally and are rarely attached to the membranes.
In purple bacteria thylakoids develop as deeply infolded regions of the plasmamembrane and are in continuation with plasmalemma.
The stroma has enzymes which concern fixation of CO2 and its conversion to carbohydrates, fats and proteins. The stroma also contains some DNA and ribosomes. Chloroplasts reproduce themselves independently of the nucleus and their ribosomes can synthesize chloroplast proteins.
Chloroplasts or chromatophores contain pigments which convert the light energy into chemical energy during photosynthesis. There are three types of pigments in photosynthetic cells, chlorophylls, carotenoids and phycobilins.
The structural formulae of some of them are given in Fig. 13-4 of these phycobilins only occur in red and blue-green algae. Compared with the chlorophyll, which is situated in membrane bound organelles chloroplasts, phycobilins are in phycobilisomes.
The chlorophylls are the most important pigments which are active in photosynthesis. They are often types: chlorophylls, a, b, c, d and e; bacteriochlorophyllsa, b, c, and d and chlorobium chlorophyll (bacteriovirdin).
Chlorophylls a and b are seen in all autotrophs except pigment containing bacteria. Chlorophyll b is also absent in the blue-green, brown and red algae. The chlorophylls c, d and e are encountered only in algae and in combination with chlorophyll a.
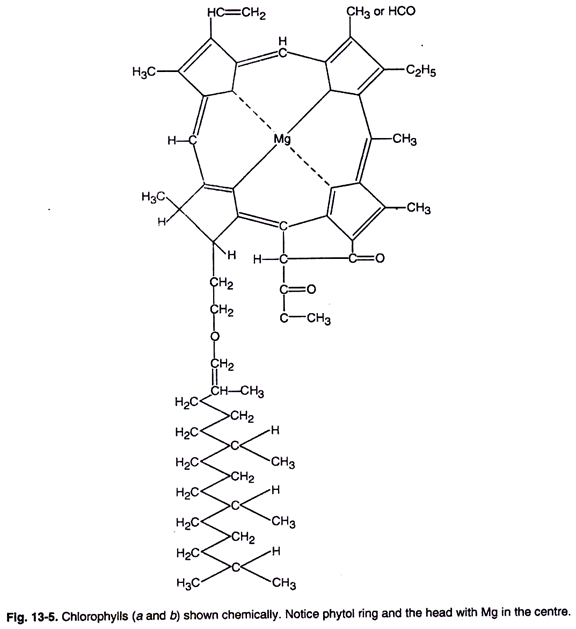
The chlorophyll molecule has a cyclic tetrapyrrolic structure (porphyrin) with an isocyclic ring containing a magnesium atom at its centre (Fig. 13-5). As will be observed a pyrrole molecule contains a skeleton of four carbon and nitrogen and has a ring structure.
Four such pyrroles arranged in a ring form the head (the porphyrin part) of a chlorophyll molecule (Fig. 13-5). From one of the pyrrole rings at 0 atom is a long chain alcohol, the phytol part of the molecule, forms its “tail”. The isocyclic ring is a 5-carbon ring and is the chemically reactive site of the molecule (Fig. 13-5).
Different types of chlorophylls have different substitution in the pyrrole ring of porphyrin (Fig. 13-4).
 Chlorophyll a is blue green, while chlorophyll b is yellow-green in colour, respectively. In chlorophyll a at carbon 3 atom, a methyl group is present while in chlorophyll b an aldehyde group is present.
Chlorophyll a is blue green, while chlorophyll b is yellow-green in colour, respectively. In chlorophyll a at carbon 3 atom, a methyl group is present while in chlorophyll b an aldehyde group is present.
Chlorophyll a is soluble in petroleum ether, while chlorophyll b is best soluble in methyl alcohol. Both chlorophyll a and b show an absorption maximum in the blue-violet region with peaks at about 429 nm and 453 nm respectively. Chlorophyll a and b have a secondary absorption maxima in the red region with peaks at about 663 nm and 645 nm, respectively.
When in solution chlorophyll is highly fluorescent and emits a deep red colour on illumination. In intact plants they do not show any fluorescence.
The empirical formula of the two chlorophylls is given below:
Chlorophyll a = C55 H72Os N4 Mg
Chlorophyll b = Cs5 H7O O6 N4 Mg
Chlorophyll Synthesis:
Several investigators have unfolded most of the steps concerning synthesis of chlorophyll a.
Some of the suggested steps are as follow:
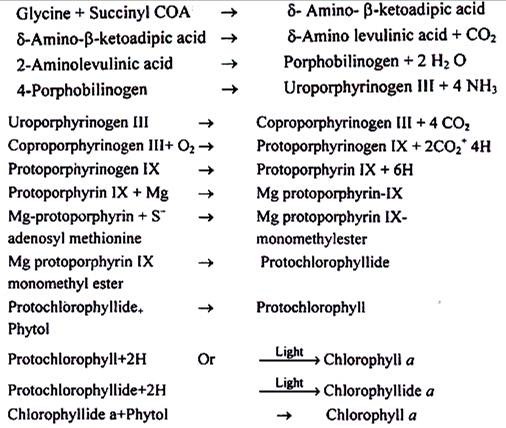
(a) Succinyl COA, an intermediate of Krebs cycle combines with glycine amino acid to form δ- amino β- Ketoadipic acid as unstable compound. This loses CO2 to yield aminolevulinic acid. The presence of cofactors pyridoxal phosphate and iron are essential. The enzyme δ- aminolevulinic acid synthetasecatalyses it. As mentioned earlier, iron deficiency causes chlorosis of young leaves. Light is shown to mediate the condensation of these two compounds.
(b) In the next step two molecules of δ-aminolevulinic acid condense, and the process is mediated by the enzyme δ-aminolevulinic acid dehydrase, to form porphobilinogen. In this reaction there is a fusion of two molecules.
(c) Then 4 molecules of porphobilinogen condense to form uroporphyrinogen III. Four ammonium ions are lost in this reaction and the process is mediated by the enzyme uroporphyrinogen-Isynthetase and uroporphyrinogen III cosynthetase.
(d) The four acetic acid substitutes of uroporphyrinogen-III yield coproporphyrinogen-III and the reaction is catalyzed by uroporphyrinogen decarboxylase.
(e) Under aerobic conditions, coproporphyrinogen-III, in the presence of coproporphyrinogen oxidative decarboxylase gives rise to protoporphyrinogen IX.
(f) Protoporphyrinogen IX undergoes oxidation and thus protoporphyrin IX is formed. It takes magnesium to form Mg protoporphyrin IX. Mg protoporphyrin methyl esterase catalyzes the addition of a methyl group of Mg protoporphyrin IX. It may be mentioned that the methyl group is donated by S-adenosyl methionine.
(g) In the next step, Mg protoporphyrin IX mono-methylester is converted to protochlorophyllide.
(h) A phytol group is added to protochlorophyllide to produce protochlorophyll. Once it was believed that protochlorophyll is the immediate precursor of chlorophyll a. However, recent evidences suggest that the immediate precursor of chlorophyll a is chlorophyllide a. When the etiolated seedlings are subjected to light, protochlorophyllide is reduced to form chlorophyllide a. The light is essentially required for this conversion.
(i) In the final step esterification of a phytol group to chlorophyllide a occurs and so chlorophyll a is produced. Enzyme chlorophyllase is involved in the process.
In gymnosperms, some ferns, and many algae, chlorophyll can be synthesized in die dark solely through enzymatic activity. On the other hand, it is believed that chlorophyll b is formed from chlorophyll a. Some of the minerals like manganse, potassium, zinc, copper, magnesium, iron, and nitrogen are essential for the synthesis of chlorophyll.
When absent or deficient they cause chlorosis. Chlorophyll formation is also dependent upon genetic factors as well. Absence of the gene(s) essential for its formation in the genetic constitution, produces seedlings from the germinating seeds which lack chlorophyll. These are known as “albinos”.
Steps in Chlorophyll Synthesis are summarized below:
Photoreceptor # 7. Carotenoid Pigments:
Carotenoids are lipid compounds which are universally present in nearlly all the higher plants and several microorganisms. They are usually red, orange, yellow or brown and are associated with chlorophyll. They are divided into two chemical groups; the carotenes and the xanthophylls. The carotenes are orange red while xanthophylls are yellow in colour. All of them have 40 carbon atoms.
Carbonates are known to occur in two isomeric forms i.e., carotene and a-carotene. Both are hydrocarbons having molecular formula as C40 H56. On the other hand, the xanthophylls, such as lutein and zeaxanthin, posses oxygen atoms united to the terminal rings. Carotene was isolated from carrot roots and lycopene which is a red pigment, from tomato fruits.
The carotenoids are composed of two six-membered rings with a highly unsaturated straight chain stretched between them. They are composed of eight, isoprene units, in which these are joined in a head-to-tail fashion (Fig. 13-4).
One of the suggestions is that the chlorophylls and carotenoids are attached to the same portion forming a water-insoluble protein complex.
Both the carotenes and xanthophylls are synthesized from acetyl coenzyme A, through the mevalonic acid pathway. At least 4 stages can be recognised in the biosynthesis of carotenoids.
These are as follow:
(i) Tail-to-tail addition of two units of geranylgeranyl pyrophosphate to form 40C skeleton called phyton.
(ii) In a series of steps, phyton is dehydrogenated to form lycopene.
(iii) Lycopene undergoes cyclization.
(iv) Xanthophylls are derived from carotenes by the oxidation.
Role of Carotenoids:
The carotenoids perform functions in diverse processes including photosynthesis, phototropisms and protection against excessive light. Their role in photosynthesis appears to be secondary since tissues rich in carotenoids and lacking chlorophyll do not photosynthesize.
It is also believed that light energy absorbed by them is transferred to chlorophyll and utilised in photosynthesis. When a plant is exposed to wavelengths of light absorbed exclusively by the carotenoids, a red fluorescence of chlorophyll a is observed.
Action spectrum and absorption spectrum for the green alga (Ulva) shows that photosynthesis is appreciable in blue-green region at 480-500 nm wavelengths, indicating some transfer of energy from the carotenoids to chlorophyll.
They also prevent destruction of chlorophyll from the degradative effects induced by excessive light and molecular oxygen (photooxidation).
They may also act as photoreceptors for light causing phototropism.