Light is a form of radiant energy, a narrow band of energy within the continuous electromagnetic spectrum of radiation omitted by the sun.
It is known since long that for photosynthesis energy is obtained from light. In actual practice light is a mixture of different colours and the electromagnetic spectrum comprises radiations of different wavelengths. The visible spectrum ranges from 3800-7800 Å and is a small region of the spectrum of the electromagnetic radiations.
There are two theories regarding light: one of these is wavelength theory which assumes that each colour of the spectrum comprise a different wavelength. On the one end of the spectrum is red light while on the other end is violet light. Longer the wavelength lesser is the energy.
On the contrary, short wavelengths have more energy. Accordingly red light will convey less energy compared with the violet light. The second model, assumes that light comprises tiny particles or photons and the theory is called quantum theory.
In the following a brief resume of energy contained in one mole of quanta (biochemical equivalent) in calories for various wavelengths is given (Table 13-2).
The absorption of light at different wavelength is called absorption spectrum. When the light is passed through an extract of chlorophyll it is possible to measure the absorption at each wavelength.
The main light absorbing pigment is chlorophyll a and it shows that the light is chiefly absorbed in the blue and red region (Fig. 13-6). Very little amount of light is absorbed in other colours. Being green in colour, chlorophyll reflects green light.
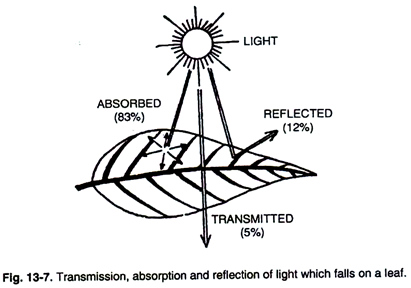
The light which falls upon the leaf is either transmitted, absorbed or is reflected (Fig. 13-7).
When a photon of light hits a chlorophyll molecule, it can transfer its energy to the outer electron, which becomes excited and is raised to a higher energy level. The molecule is made unstable. The activated electron is now trapped by various electron acceptors. In other words, chlorophyll coverts light energy into chemical bond energy and this is stored in ATP.
Chlorophyll participates actively in photosynthesis by absorbing wavelengths of light which are very effective in bringing about the process. These wavelengths have sufficient amount of energy to lift chlorophyll to a higher state of energy Fig. 13-7A.
Two major peaks are found one in the red and the other one in the blue. The action spectrum resembles more closely the absorption spectrum of the chlorophylls than of any other pigment indicating the role of chlorophylls in absorbing light energy causing photosynthesis.
Energy Relations of Photosynthesis:
For the reduction of one mole of CO2 to CH2 O with the help of water about 110 kilocalories are required. One mole quanta of red light has 43 kcal of energy. This is about one-third of requirement. In other words about 3 photons would be required to accomplish this.
One mole quanta of blue light is nearly 63.4 kcal i.e., it is about 1.4666 times that of red light. This amount is also low to reduce one CO2 molecule. Thus 8-10 photons of blue or red light are required and this number is referred to as the quantum requirement for photosynthesis.
Experiments have also demonstrated that each molecule of chlorophyll absorbs a photon only about every eight minutes. If eight photons were to be absorbed before causing a reaction, an hour would be needed before photosynthesis could occur after turning on the lights.
Experiments show that the process begins immediately after illumination. It may be remembered that the life time of an excited chlorophyll molecule is little less than 0.01 second and may be as low as 10 sup second.
The natural question is how could as many as 8-10 quanta be absorbed by a single chlorophyll molecule to cause photosynthesis. The possible answer lies in the fact that the energy photons absorbed by several chlorophyll molecules are quickly passed on to a common reaction centre.
It is at this centre that this energy is accumulated and oxygen is released without a lag. Pigment system I P 700 is one such reaction centre. In summary, several chlorophyll molecules collaborate to harvest energy. The light quantum migrates from one chlorophyll molecule to another. Ultimately energy is transferred to P 700 chlorophyll molecule and it is oxidized.