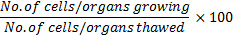In this article we will discuss about the yeast used in bakery foods:- 1. Effect of Ingredients and Processing on Yeast Performance 2. Forms of Yeast Used in Baking 3. Use of Yeast in Special Dough Systems 4. Performance and 5. Determination.
Contents
Effect of Ingredients and Processing on Yeast Performance:
Recent studies have dealt with a larger number of variables which affect yeast activity in doughs. These variables are treated separately because this permits a better understanding of the manner in which they influence yeast performance.
In general, fermentation activity is increased by higher yeast concentrations, by higher fermentation temperatures, and by the addition of sugars up to 4-6% based on the weight of flour. Fermentation activity is decreased by sugar concentrations above 6%, by increased salt concentrations, by pH values below 4.5, and by the addition of mold inhibitors.
The use of oxidants and other dough conditioners affects the elasticity of doughs and the permeability of doughs to carbon dioxide gas. This affects the amount of CO2 retained in the dough and, consequently, the leavening effect of the yeast. But oxidants and most dough conditioners have little or no effect on the fermentation activity of yeast per se, and, therefore, they will not be discussed below.
It may be noted in passing that recent work by Bell et al. (1977) indicates that dough permeability appears to be related to the well-known improving effect of fat in dough. During the early baking stage, when the loaf is rapidly expanding, fat-containing doughs exhibit more carbon dioxide retention than doughs made with no added fat.
Fermentable Sugars:
Under the anaerobic conditions prevailing in dough, yeast ferments sugars to ethanol and carbon dioxide. These sugars are the mono-saccharides glucose and fructose and the disaccharides sucrose and maltose. Lactose is not fermented by bakers’ yeast.
Starches and dextrins are not fermented by yeast but may serve as sources of fermentable sugars if they are hydrolyzed by amylases. Flour contains from about 0.3 to 0.5% of fermentable sugars. In traditional doughs which consisted of water, flour, yeast, and salt and in today’s lean doughs, the rate of gas production follows a double humped curve as shown in Fig. 8.8.
The relatively high rate of gas production at 30 min represents the fermentation of sugars as they pre-exist in the flour. The second increase in gassing rate occurs after 60 – 90 min and corresponds to the liberation of maltose from the starch of the flour by amylases. The final drop of the rate after 2% hr reflects the exhaustion of the supply of fermentable sugars.
Flour contains both α- and β-amylase but the concentration of α-amylase is quite low and limits the formation of maltose. Therefore, malt, which contains sufficient α-amylase, is generally added to flour before delivery to the baker fungal α-amylase may be used.
At times, the baker may further supplement his doughs with either malt or fungal amylase, in the production of certain items. The rate of hydrolysis of raw starch in flour is quite slow and only so-called damaged starch granules can be hydrolyzed enzymatically. The amount of damaged starch accounts for about 5-8% of the weight of the flour. This means that the total amount of sugar ultimately available for fermentation is limited unless additional sugars are added to the dough.
In lean doughs maltose is the principal fermentable sugar. Therefore, it is important to use a strain of bakers’ yeast with good “malto-zymase” activity. This enzyme complex has been thought of as a group of enzymes capable of hydrolyzing maltose to glucose and of fermenting glucose via the glycolytic pathway. It is now apparent that yeasts contain sufficient internal maltase (glucosidase) to hydrolyze maltose quickly.
At present it is believed that transport of maltose into the yeast cell is the limiting step in maltose fermentation, and the presence of an active transport mechanism catalyzed by a “maltose permease” has been assumed. Some yeast strains contain the required enzyme system constitutively.
Others have to be adapted to the fermentation of maltose. While the fermentation of maltose has been of great practical significance in the past, it has lost importance because of the addition of fermentable sugars to doughs. Even lean doughs contain from 0.5 to 2% added sugar in the United States.
Only a few investigations have been carried out on the actual levels of sugars in fermenting doughs. Figure 8.9 shows the sugar levels of dough made from a liquid pre-ferment at the beginning of the pre-ferment period at the end of the pre-ferment period, and in the final bread.
The original source of sugars was a corn syrup which contributed 8% fermentable sugar (3.9% maltose and 4.1% glucose). Glucose is rapidly fermented throughout the fermentation period. Maltose is fermented slowly in the pre-ferment. In the dough the level of maltose actually increases because the rate of maltose formation from starch is greater than the rate of fermentation.
Therefore, the final bread contains hardly any glucose but almost 4% maltose. The fate of sugars in straight dough has been demonstrated by Koch et al. (1954). In his tests, 1 g of yeast solids fermented about 1.2 g of sugar per hour. The compressed yeasts available in the United States ferment about 2.5 g of sugar per g of yeast solids per hour in straight doughs and lean doughs, and about 1 g of sugar in sweet doughs.
Residual bread sugars in laboratory-produced sponge dough bread made with several different sweetener types are shown in Table 8.9. These previously unpublished data indicate, again, that where both fructose and glucose are present in dough, glucose is more rapidly fermented.
Maltose levels are low (0.7 to 0.9%) if no maltose is added as a part of the sweetener system. The residual lactose, of course, is derived from the usage of dairy ingredients in the formulation. Taste panel comparisons of these breads suggest that the first 4 breads (made with either 10.0% dextrose, 6.7% sucrose, 10.5% high fructose corn syrup, or 95 D.E. com syrup) had approximately the same level of residual sweetness.
Effect of pH and Temperature:
The activity of bakers’ yeast is almost constant over a pH range from 4 to 7. This is also the range for various doughs used in the industry with the exception of sourdoughs. Below a pH of 4 activity drops sharply, and above a pH of 7 the drop off is gradual. The relative insensitivity of yeast to a 300-fold range of hydrogen ion concentrations is due to the fact that the internal pH of the yeast cell is maintained fairly constant over the entire range. The pH near the center of the cell is approximately 5.8 but it differs for different structures within the cell. Gassing rates at various pH levels have been determined by Franz (1961), Seeley and Ziegler (1962), and Garver et al. (1966).
In liquid pre-ferments which do not contain flour or nonfat dry milk solids, the pH drops during fermentation because of the production of carbon dioxide and organic acids by yeasts and lactic acid bacteria. Buffer salts must be added to such pre-ferments to keep the pH above 4.5.
Bakery sponges are usually set at a temperature of 24°-26°C and there is a rise of 3°-4°C during the sponge fermentation. Dough temperatures are generally somewhat higher and in the case of continuously mixed doughs may reach 35°C. Almost all bakery fermentations are carried out within this range of 25°-35°C.
These temperatures are convenient for bakery operations and the exact temperatures are chosen to produce doughs with suitable elasticity and handling characteristics and to permit optimum bread quality. These temperatures do not provide optimum gas production rates.
There have been only a few exacting studies on the effect of temperature on gas production rates. Available data indicate that the fermentation rate increases by a factor of 1.5 to 2 for a 10°C increase in temperature. Figure 8.8 shows that there is a 50% increase in the rate of fermentation if the temperature is raised from 27.5°C to 32.5°C. The rate of CO2 evolution in straight doughs increased twofold from 20° to 27°C.
During the early stages of baking the volume of the loaf increases considerably. This is due to thermal expansion of the entrapped gas of the dough, to the formation of additional CO2 because of its decreased solubility in the dough water, and to the production of additional CO2 by fermentation. It is difficult to estimate how much of this so-called “oven spring” is due to fermentation.
There is about a 10 min period in the oven before the center of the loaf reaches a temperature of 55°C, the temperature at which yeast cells are killed rapidly. Figure 8.10 shows the rate of kill at temperatures of 48°, 50° and 52°C. There is a considerable lag (also temperature dependent) before the curve follows first order reaction kinetics.
Osmotic Pressure:
Yeast fermentation is strongly inhibited at high osmotic pressures in doughs. The major contributors to osmotic pressure in doughs are salt and sugars. At salt concentrations up to 1.5% there is little inhibition in doughs, but at concentrations of 2-2.5% which are common in bread doughs there was considerable inhibition. With sucrose, glucose, maltose, and fructose, inhibition becomes apparent at concentrations exceeding 4-5%.
Yeasts with a high invertase activity are more inhibited by high sucrose concentrations than yeasts with low invertase activity. This is probably due to the increase in osmotic pressure when sucrose is hydrolyzed by the enzyme.
Yeasts vary greatly in their tolerance to high osmotic pressure. This tolerance is a function of the strain but it also depends to a considerable extent on the conditions under which the yeast is grown. Good osmotolerance is particularly important for yeast raised sweet goods which may contain 20-25% of sugar based on the weight of the flour. Table 8.10 shows that for bakers’ compressed yeast the rate of gas production was only about 35% of that in lean doughs.
Fermentation Inhibitors:
Ethanol is a strong inhibitor of yeast growth and yeast fermentation. At ethanol levels exceeding 4% (weight per volume) there is some inhibition of the rate of ethanol formation and carbon dioxide evolution. For each g of sugar that has been fermented about 0.45 g of ethanol is formed.
Sponge doughs and straight doughs have been reported to contain 3 and 1.5% ethanol, respectively, and liquid pre-ferments from 1.5 to 1.75%. At such levels the inhibiting effect of ethanol is minimal. It is not negligible in concentrated pre-ferments.
Cole et al. (1962) reported the presence of 1.8, 3.3, and 6.8% ethanol (by vol.) in pre-ferments containing 3.2, 6.6, and 11.9% sucrose, respectively. Most of the ethanol formed by fermentation is driven off during baking so that a freshly baked loaf may contain no more than 0.8% ethanol based on the weight of flour.
Mold inhibitors are commonly added to commercial white bread. This is particularly important if the bread is sliced before wrapping since additional surface area is exposed to air. Propionates are the most widely used inhibitors, and levels of 0.3% of sodium or calcium propionate are frequently used. Other suitable mold inhibitors are sodium di-acetate and vinegar.
There are considerable differences in the degree of inhibition reported by various authors because other variables such as pH may affect the degree of yeast inhibition. Schulz (1967) reported the highest rate of inhibition and also found that maltose fermentation was more strongly inhibited than the fermentation of sucrose or glucose. His tests had been done with lean formulae. At a level of 0.25% propionate, an inhibition of the rate of fermentation by 20% can be expected.
Effect of Yeast Nutrients:
For doughs which undergo very short fermentation periods the addition of extra nutrients is not required. But for normal fermentation periods the addition of a readily assimilated nitrogen source is useful although there is little growth of yeast during the fermentation.
Such nitrogen and additional minerals are usually added in the form of “yeast foods.” Such yeast foods contain not only yeast nutrients but also oxidants and sometimes salt which adjust the pH of the dough. Yeast foods generally contain about 10% of either ammonium chloride or ammonium sulfate as a source of nitrogen, potassium bromate and/or iodate as oxidants and monocalcium phosphate for pH adjustment if the pH of the water is alkaline. Flour, salt, and calcium sulfate are often used as fillers. A normal level of addition of such a yeast food is 0.5% based on the weight of flour.
Addition of minerals is rarely required. If very soft water is used for the makeup, the addition of calcium salts is desirable. Schultz et al. (1942) in their classical paper found that the stimulating effect of flour on fermenting activity was due to thiamin. This is important for liquid pre-ferments which contain no flour and which consequently require thiamin. For this reason sufficient thiamin is usually added during the production of bakers’ yeast to supply at least 50 μg of thiamin per g of yeast solids to the compressed yeast.
The oxidants and the monocalcium phosphate do not affect yeast activity directly. However, they serve to improve gas retention in doughs and hence improve the leavening effect of the yeast. This is the rationale for the combined use of yeast nutrients and oxidants. For a more detailed discussion of yeast foods see also Reed (1972).
Forms of Yeast Used in Baking:
Determination of the Activity of Bakers’ Yeast:
A laboratory bake test is accurate in the sense that it reflects performance of the yeast in bakery operations. In one such test straight doughs yielding 20.45-kg (1-lb) loaves were produced. In such tests one measures either the time required for dough to proof to a given height or one measures the volume of the bread for a given proof time.
Such tests may also be carried out on a smaller scale, for instance, with “pup” loaves requiring only 100 g of flour for each test. There are two basic problems with this type of test. First, the bake test is imprecise. Its results depend on the skill of the operator, on the type of flour used, on the temperature of the bake shop, and on the proper functioning of mixers, proof cabinets, molders, and ovens.
The second problem regards the great variety of uses of yeast in a bakery. A particular bake test reflects accurately only that operation which it imitates on a laboratory scale. But bakers produce bread from straight doughs, sponge doughs, lean doughs, sweet doughs, doughs made with liquid pre-ferments, or various combinations of these. Therefore, it is almost impossible to reflect all of the variations in bakers’ processes in one or several simple bake tests.
The alternative is a simple determination of the fermenting power of the yeast by measuring the amount of carbon dioxide evolved in a given time period. This can be done in solutions of various sugars in a simple fermentometer such as the one described by Schultz et al. (1942).
Such simple gas measuring devices are still in use and a suitable arrangement for determining the activity of wine yeast has recently been described. With such tests one can determine the amount of carbon dioxide produced by bubbling the gas through an alkaline solution and by back- titration. Or, one can measure the amount of gas volumetrically at atmospheric pressures or one measures the pressure of the gas in a defined volume. These simple tests have a serious drawback. The osmotic pressure in doughs is very much greater than in simple sugar solutions, and yeasts whose activity is greatly reduced by higher osmotic pressures vary greatly in their osmotolerance.
For these reasons it is advisable to measure carbon dioxide evolution in actual doughs. Since total gas evolution is measured and not the amount of gas remaining in the dough it is not essential that doughs be mixed to a given degree of elasticity. Such dough pieces weighing from 10 to 100 g can be introduced into hermetically sealed cups with pressure meters.
More frequently they are placed into instruments which measure the volume of total evolved gas at normal atmospheric pressure. A suitable instrument is the S.J.A. fermentograph which facilitates the measurement by an automatic chart of gas evolution over a given time period. Figure 8.11 shows such a Fermentograph curve.
Doughs may also be prepared and inserted into pressure cups. The gassing power is then expressed as mm Hg pressure. Shogren et al. (1977) used such a system with a conventional straight dough containing flour, 100%; skim milk solids, 4%; sugar, 6%; salt, 1.5%; malt, 0.25%; compressed yeast, 3%; and 20 ppm of KBrO3.
All percentage values are expressed as percentages of the flour used as is common in formulations of baked goods. Figure 8.12 shows the results of such gassing power tests when the percentage of water was varied from 40 to 200% (based on flour). A normal percentage of water would be in the 60-70% range.
Gassing power increases greatly at higher levels of absorption (percentage of water). While the authors explain this on the basis of additional nutrients leached from the flour, it is more likely that the reduced osmotic pressure of the doughs at higher concentrations of water accounts for the increased gassing power of the yeast.
An interesting method which involves the preparation of doughs but avoids the variability of flour has been developed by Schulz (1972). This starch dough method as modified by Briimmer (1977) requires the mixing of a dough consisting of 400 ml of water, 500 g of cornstarch, 15 g carob flour, 25 g sucrose or any other sugar, and either 12.5 g of compressed yeast or 3 g of active dry yeast. Dough pieces weighing 400 g are placed into 2 liter measuring cylinders and the volume is measured every 15 min for a period of 150 min.
Not all of the carbon dioxide evolved by the fermentation of sugars remains entrapped in the dough. A certain fraction of the gas escapes and does not serve as a leavening gas. The amount of gas that escapes from the dough depends on the strength of the flour and on proper development of the dough.
For this reason any method which measures total gas development by a yeast is valid only if one assumes the yeast does not affect the permeability of the dough membrane for carbon dioxide gas. This assumption is probably justified for compressed yeast; it is not always justified for active dry yeast if leached yeast solids (mainly glutathione) affect the rheology of the dough.
The results of baking tests are often expressed in terms of proof minutes, and the results of gassing power tests in terms of ml of CO2 evolved. These are arbitrary expressions and do not permit a comparison of the data obtained in different laboratories. It is more meaningful to express yeast activity on the basis of the millimoles of CO2 evolved per hour and per gram of yeast solids. Such values will generally vary between 10 and 25 mM of CO2/hr/g yeast solids, depending on the type of yeast and the particular dough composition.
Typical values for U.S. compressed yeast and active dry yeast for 3 types of dough systems are shown in Table 8.10. One can relate these values to the amount of sugar fermented by a given amount of yeast. Ten mM of CO2 are evolved by the fermentation of 0.9 g of glucose. Therefore, 1 g of yeast solids which leads to the production of 10 mM of CO2 will have fermented 0.9 g of this sugar (compressed yeast contains 30% solids).
Compressed Yeast:
The cut kilograms (lb) of compressed yeast are wrapped in wax paper and packaged 22.7 kg (50 lb) to a case. The cases are shipped by refrigerated trucks either directly to the bakery or are held refrigerated in distribution centers for later delivery to bakers. In the United States, deliveries to bakers are made every other day, twice weekly, or once a week depending to some extent on the distance from the yeast factory. While bakers request shipment of the freshest yeast it is probably more important that the yeast be properly cooled before shipment and that it be shipped to the bakery and stored in the bakery so that its temperature does not exceed 5°-8°C.
Refrigeration of yeast in the bakery is particularly important with regard to yeast that may have been brought to the mixer floor and that may not have been used during a shift. If this yeast is allowed to warm up, it may not be possible to cool it down again because respiration of the yeast at temperatures above 20°C makes it difficult to do so effectively.
For larger bakeries and especially for bakeries using liquid pre-ferments, the yeast press cake is usually crumbled at the yeast factory and packed in 22.7 kg (50 lb) bags with polyethylene inner liners. The same precautions have to be used with regard to this crumbled yeast as to the compressed yeast cakes.
In the past it has been customary to disperse compressed yeast in buckets with water before adding it to the mixer. This is not necessary and compressed yeast cakes can be added directly to the flour in high speed mixers. For the preparation of liquid pre-ferments it is customary to suspend the yeast and other minor ingredients in a mixing tank from which it is pumped to the pre-ferment tank.
In some larger bakeries yeast slurry tanks have been installed. Yeast is suspended in an equal weight of water. The slurry is kept at 5°- 10°C with slight agitation to prevent settling of the yeast. From the slurry tank the desired amount of yeast can be pumped directly to the mixer.
The above conditions for refrigeration of compressed yeast apply particularly to the United States where yeast is often shipped over distances up to 1500 km, and where very fast fermenting yeast is required. In some other countries yeast of a different strain and of somewhat lower nitrogen content can be shipped without refrigeration.
For use by consumers and for sale through grocery stores, compressed yeast is packaged in 18 g and 56 g weights, wrapped either in aluminum foil or in wax paper. This yeast generally has lower nitrogen content and about 10% of starch has been added. Both of these measures assure a better shelf life which exceeds several weeks. Nevertheless, the development of mold on yeast cakes is a problem when turnover in the stores is slow. For consumer use compressed yeast has been largely replaced by active dry yeast.
Active Dry Yeast:
In general, active dry yeast has not replaced compressed yeast in wholesale bakeries. This is readily apparent from the figures shown in Table 8.10. These indicate that for a given amount of yeast solids, active dry yeasts ferment more slowly than compressed yeasts in regular doughs and lean doughs. However, there is some use of active dry yeast in sweet doughs reflecting the good fermenting activity at higher sugar levels.
In the United States, active dry yeast is shipped to bakers in fiber drums with polyethylene liners. It has a useful storage life of up to 3 months at ambient temperatures and up to 6 months if it is kept refrigerated. For export and for storage for prolonged periods the yeast is packaged in 11.3 kg (25 lb) cans which are flushed with nitrogen gas to replace the air atmosphere.
For smaller 0.9 kg (2 lb) cans it is simpler to apply a vacuum. In either case the cans must be hermetically sealed. Alternatively, the yeast may be packed in flexible packaging material under vacuum or a carbon dioxide atmosphere. All of these yeasts have a useful shelf life of at least 1 year provided the seal is not broken.
In the United States, active dry yeast has largely replaced compressed yeast for sale to institutions such as restaurants, schools, prisons, etc., and for sale to consumers through grocery stores. Active dry yeast is also preferred in countries where a hot climate or lack of refrigerated facilities makes it difficult to distribute compressed yeast satisfactorily.
Air lift dried “instant” yeasts which have recently come on the market are packed in flexible, hermetically sealed pouches or bags. They have a relatively high fermenting activity which is intermediate between that shown for compressed yeast and active dry yeast in Table 8.10. These yeasts have the same excellent stability as long as they are protected by an inert atmosphere. They have very poor stability once the seal has been broken and air has been admitted.
Consumer active dry yeast is usually packaged in smaller aluminum foil pouches (7 g per pouch). These pouches are flushed with nitrogen gas and heat sealed. The storage life is also at least 1 year. However, these packages are subject to mechanical shock during shipment and particularly during handling in grocery stores. The development of small leaks in the sealing area is not uncommon. This results in an accelerated loss in yeast activity. For this reason the reliability of the hermetic seal is more important than differences in the original fermenting activity of the yeast.
For use in baking active dry yeast is generally rehydrated in water of about 30°-40°C before addition to the mixer or to the pre-ferment tank. A rehydration period of 5 min is adequate to obtain good dispersion and rehydration. If the yeast is finely ground it can be added directly to the; flour in the mixer. The air lift dried “instant” yeasts are particularly suitable for direct addition to the flour without prior rehydration.
Doughs made with active dry yeast are slacker, more extensible, and more relaxed than doughs made with compressed yeast. This is .due to the leaching of a reducing compound, glutathione (GSH), into the water used for rehydration. Direct addition of the yeast to the flour prior to addition of the dough water minimizes this effect but does not eliminate it.
Ponte et al. (1960) has shown that the slackening effect of ADY is indeed due to GSH by separating rehydrated ADY from the rehydration water. If the rehydration water is discarded there is no slackening effect. However, this separation is not practical for commercial operations.
As the result of the presence of GSH in rehydrated ADY the mixing time of doughs is reduced by about 25%. The slackening effect is beneficial for doughs made from very strong flours, for pizza doughs, bun doughs, and some sweet doughs where a well- relaxed dough is desired. In many other systems the slackening effect is not desired and in that case it can be counteracted by increased oxidation. Figure 8.13 shows the oxidation requirements of straight doughs for compressed yeast and active dry yeast.
Special Active Dry Yeast Preparations:
Throughout the past 50 years there have been numerous attempts to dry compressed yeast together with such materials as starches, flour, inorganic salts, and others. In general these have not been successful. In some cases an attempt has been made to include other dough ingredients with ADY products.
For instance, Distiller’s Co., Ltd. (1976) has patented a product which contains ADY, an edible oil, L-cysteine, and azodicarbonamide. The latter two compounds are dough conditioners. Hartmeier (1976) used maltodextrin as a carrier for the drying of compressed yeast and added grape or fruit syrups prior to drying.
Normally, ADY contains no additives. For ADY products of moisture values below 6%, an emulsifier such as sorbitan-monostearate is generally added to facilitate rehydration and to minimize the leaching phenomenon. A “protected ADY” of improved stability can be obtained by adding the emulsifier and about 0.1% of an antioxidant, butytated hydroxyanisole, to ADY of low moisture. Such yeasts are commercially available and used in some instances as consumer yeasts.
Concentration of Yeasts in Doughs:
Bakers’ compressed yeast contains between 25 and 35 x 109 cells per g. The total number of cells depends, of course, on the size of the cell, and for smaller cells the number of cells per g is higher. In normal sponge dough the number of yeast cells is about 300 to 400 x 106 cells per g.
There is little or no multiplication of cells during the 3 to 4 hr sponge period but the number of budding cells increases from 30 to 50%. This is true only because a large number of cells have been added in the form of bakers’ compressed yeast. For small concentrations of yeast, growth is considerable during long fermentation periods (overnight).
The actual concentration of compressed yeast used commercially varies with the type of dough system and with the desired proof time. Generally proof times are between 45 and 60 min. Finney et al. (1976) used a straight dough procedure. They determined optimum bread quality by varying total fermentation time (exclusive of proof time) for varying yeast concentrations. Their results are shown in Fig. 8.14.
The general shape of the curve is similar to the early work by Fisher and Halton (1937). It is apparent that the effective fermentation time cannot be reduced beyond a certain limit no matter how much compressed yeast is used, that is, if one wishes to produce bread of excellent quality. When the fermentation time was decreased from 180 to 70 min, the yeast concentration had to be increased from 2 to 7.2% and the requirement for bromate addition was tripled. Proof time decreased from 55 to 21.5 min.
Table 8.11 shows the levels of compressed yeast customarily used in various dough systems and for the production of various baked goods. In some instances the range of concentrations used in practice is quite small. This is particularly true for sponge dough breads and breads made with continuous mix processes.
For various sweet doughs and frozen, unbaked doughs the range is quite large, reflecting variations in dough composition and in processing conditions. The interdependence of some of these variables is shown in Table 8.12 which indicates the requirement for larger yeast concentrations for liquid pre-ferments with lesser amounts of flour in the pre-ferment.
Use of Yeast in Special Dough Systems:
Short Time Doughs:
Such doughs are often used when it is important to reduce the overall time required for bread processing. Short time doughs may yield bread in about 2 hr, as opposed to 7-8 hr for conventional sponge doughs. Retail bakers and food service operators utilize short time doughs to avoid night and early morning working hours, and to reduce labor costs. Large wholesale bakers do not employ short time doughs in their operations. Sometimes such doughs are incorrectly called no-time doughs.
Baked goods of reasonable quality can be obtained with short time doughs, but higher levels of yeast and oxidants, warmer dough temperatures and increased dough mixing are required, and the use of cysteine or other agents to relax the dough is sometimes advisable. The more important problems with short time doughs include decreased product shelf life and poorer processing tolerance.
Decreased shelf life is not as serious a problem with retail bakers or food service operations as it is with wholesale bakers. Lessened processing tolerance (i.e., temperature, dough elasticity, timing, etc.) is acceptable to the small baker, but not to the large, heavily mechanized baker. It is often difficult to achieve proper proof height for short time doughs in the normal 55-60 min anticipated by the baker.
Finney et al. (1976) were able to obtain bread of equal quality for the conditions shown in Table 8.13. The interrelationship among fermentation time, proof time, and oxidation requirement is quite apparent. The principles which follow from this relationship have been well established. That is, a decrease in fermentation time calls for an increase in yeast concentration and a drastic decrease in proof time. Oxidation requirements are greatly increased but the absolute values depend very much on the type of flour used.
It is interesting to compare the results of this laboratory investigation with actual bakery practice as reported by Shirley (1977). Preparing short time doughs with floor times of 20 to 30 min it was necessary to increase mixing time, to use protease or cysteine (a reducing compound) to obtain full development of the dough, and to increase yeast levels.
Some additional steps had to be taken which could not be properly brought out in the laboratory procedures. Because of the very short fermentation time, the pH did not drop sufficiently and vinegar was added. Levels of sugar had to be reduced to prevent excessive browning; and the level of salt was reduced to reduce excessive proof times.
Frozen Doughs:
Yeast leavened, unbaked doughs may be preserved by freezing, either in the form of small dough slabs or in the form of formed rolls or loaves. The products are later thawed, proofed, and baked. This freezing process is used by some bakeries to ensure a supply of doughs for bake-off on weekends or holiday periods. Frozen storage for this purpose is rarely longer than 1 week.
A major market for frozen doughs is “in-store” bakeries and institutions, which bake bread on the premises but do not wish to operate the heavy mixing and make-up equipment of a bakery. For this application the production of frozen doughs presents no major problem since they can be stored at -25° to 30°C for 2-4 weeks without appreciable loss in bake activity. There is also a consumer market for frozen bread and roll doughs.
This requires a shelf life of several months and deterioration of yeast activity during frozen storage is a serious problem. This deterioration is caused by a loss of yeast viability and by changes in the structure of the dough. Loss of yeast viability is by far the more important cause of deterioration. This results in prolonged proof times and inferior internal and external characteristics of the baked goods.
Compressed yeast can be frozen and kept at temperatures between -25° and -30°C for several months without appreciable loss in viability and bake activity. Mazur and Schmidt (1968) froze yeast with extremely fast freezing rates. Freezing in fractions of a second or freezing to the temperature of liquid nitrogen (-76°C) is harmful to yeast survival.
In the production of frozen baked goods the loss in yeast viability does not occur during freezing but throughout the period of frozen storage. Figure 8.15 shows the loss of viability in straight doughs as a function of yeast concentration and fermentation time prior to freezing.
It is clear that longer fermentation periods lead to greater damage of the yeast cells. This damage can be due to- (1) an increased sensitivity of cells in a state of high metabolic activity, (2) the effect of soluble dough constituents (sugar, salt, etc.), or (3) the effect of the products of yeast metabolism, that is, CO2, ethanol, or other fermentation by-products.
Most authors are inclined to see the cause of yeast damage in the heightened susceptibility of the yeast itself. This seems to be a reasonable assumption although no experimental evidence is available to support this point of view. The second hypothesis has not been tested Hsu et al (1979A.B) have dealt with the third hypothesis.
They have shown that the volatile fraction of liquid ferments is a major factor in producing yeast damage. But not all of the damage could be attributed to the 2.5% of ethanol which had been formed prior to freezing. It must also be remembered that a concentration of 2.5% of ethanol has a small but demonstrable effect in inhibiting fermentation.
Too little attention has been paid to the effect of freezing rate thawing rate, to the temperature of frozen storage, or to possible fluctuations in the temperature during frozen storage. Hsu et al. (1979 B) have reported that freezing at different temperatures causes different levels of damage Proof times of the frozen doughs were 72, 71, and 132 min for freezing at -10° -20° and -40°C, respectively. Freezing at -78°C resulted in doughs which could not be proofed in 6 hr.
In assessing the applicability of laboratory results to commercial operations one has to keep in mind that some authors have worked with storage periods of only 2-4 weeks while others have used periods of frozen storage of several months.
Regardless of the various hypotheses which have been proposed, the recommendations for the conduct of commercial operations are quite consistent. There are- a high level of yeast (5-6%); high levels of oxidation (30-40 ppm bromate); cool doughs (18°C after mixing); and rapid conveying of the dough slabs or formed loaves into the freezer. Lorenz (1974) has summarized these recommendations as well as the earlier literature.
Complete Bakery Mixes:
Institutional bakeries and some wholesale bakeries use so-called “bakery mixes” as principal dough ingredients. Such mixes contain sugar, shortening, salt, all of the minor dough ingredients, and part or all of the flour required. They do not contain the yeast. Bakery mixes are particularly suited to the production of sweet goods, such as doughnuts. They generally require only the addition of yeast and water.
For the formulation of “complete” mixes, finely ground ADY may be added. Such mixes are stable for a limited time; generally for 2-4 weeks. A much more stable complete mix can be obtained by use of a “protected ADY.” In addition moisture pickup by the yeast from the flour must be prevented by use of low moisture flour. With flour whose moisture content had been reduced to 9 -10% the shelf life of complete mixes could be increased to 3 months, and for 8% moisture flour it could be increased to 1 year.
Better stability of complete mixes may also be obtained by packaging in an inert atmosphere. Complete mixes are available in the United States for the wholesale market, and in Japan for the wholesale and consumer market. There is also consumer market for such mixes in the United Kingdom.