In this article we will discuss about:- 1. Subject-Matter of Restriction Enzymes 2. Nomenclature of Restriction Enzymes 3. Types 4. Sites 5. Restriction Enzymes Generated Staggered and Blunt Ends 6. Purification.
Subject-Matter of Restriction Enzymes:
Restriction enzymes, also known as restriction endonucleases, have played a key role in the development of recombinant DNA technology. These have been found in microorganisms tested and are known to cut double-stranded DNA to yield restriction fragments.
The first observations on the existence of restriction enzymes was made by Arber and Dussoix in 1962, and proposed model to explain the restriction phenomenon. Their views on restriction enzymes affirmed that certain bacterial strains contained endonucleases able to cleave unprotected DNA. In addition, several other strains contained a modification system responsible for protecting their own DNA.
Some of the observations were made by W. Arber and his associates while studying the efficiency of plating of the bacteriophage lambda on different strains of Escherichia coli. They even demonstrated that restriction endonucleases were able to cleave DNA from other strains while exempting that of the original strains.
They are also associated with modifying enzymes, which methylate the DNA. Methylated DNA escape cleavage by endonucleases, and prevents the cell from degrading its own DNA. Thus, invading foreign DNA in bacteria that has not been correctly methylated will be degraded.
In 1970, Smith, Wilcox and Kelly have characterized and purified restriction enzymes and elucidated their recognition and cleavage site of a more useful restriction enzyme, Hind II.
Nomenclature of Restriction Enzymes:
The discovery of a large number of restriction enzymes led to the systematic assignment of uniform nomenclature, proposed initially by Smith and Nathens. According to the nomenclature three letter codes is given for each restriction endonucleases.
The first letter code is always a genus name and written in capital and the following letters are represented by species name. The subsequent letter designates the strain, and roman numbers indicate different endonucleases from the same organism. Example, EcoRI, EcoRII.
Types of Restriction Systems:
Three types of restriction-modification systems are recognised. These are Type I, Type II and Type III. Type I and Type III restriction/modification enzyme systems are less known and less common, but biologically very curious. They are large multimeric proteins capable of cleaving and modifying DNA. They require ATP, Mg++ for their activity.
Type II system produce the well-known restriction enzymes and they are most useful in molecular biology applications. All the enzymes recognize particular recognition sequences, but only the type II restriction systems cut within those recognition sequences, Majority of type n restriction enzymes recognise tetra, penta or hexa and even hepta nucleotide sequence (see Table 13.1).
Type II enzymes are smaller monomelic proteins that require only Mg++ for activity. They are capable of cleaving DNA at specific sites and produce fragments with cohesive termini.
Sites of Restriction Enzymes:
Restriction enzymes recognize specific sites of different lengths and base composition. The typical restriction enzyme Type II site is an exact palindrome of 4, 5, 6, 7 or 8 base pair. For example, EcoRI recognition site is GAATTC. Thus, as long as the same polarity exists recognition sites generally read the same on both strands.
Such sequences are often described as palindromes. Some other restriction enzymes do not require a palindrome for site recognition at the typically cut DNA and one side of the recognition site. Most enzymes will not cut DNA methylated on one or both strands of their recognition site, although a few require methylation in order to cut DNA.
The number and size of the fragments generated by a restriction enzyme depend on the frequency of occurrence of the restriction site in the DNA to be cut. For example, in the DNA of 50% GC ratio, a four base recognition occurs once every 256 bp (44), similarly a six base recognition site occurs for every 4,096 bp (46) and light base recognition site occurs for every 65,536 bp (48).
Construction of genomic mapping requires cutting of DNA into larger fragments by eight base cutters. Six base cutters are used for cloning into specific regions of plasmids. Different restriction enzymes can recognise the same sequence, for example, Aha III and Dra I recognize and cut DNA at TTTAAA. Enzymes with the same recognition sequence do not necessarily cut at the same position of restriction site.
Various factors can influence cutting of the DNA. The most important are methylation, structure of the substrate and the nature of buffer employed. Restriction enzyme will generally not cut molecules which are methylated at recognition site. Methylation takes place at other position within the recognition site may fail to affect cleavage.
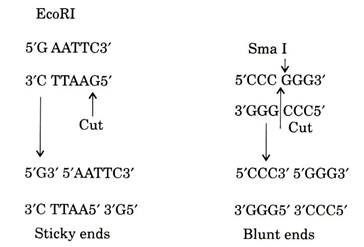
Restriction Enzymes Generated Staggered and Blunt Ends:
Cleavage by restriction enzyme can generate a number of different ends. The recognition sites of number of type II restriction enzymes often make a ‘staggered’ cut to leave molecule to generate short single-stranded ends. These are called as sticky ends. The single strand may generate either at 5′ end or to 3′ end depending on the enzyme, usually the ends have 5′ phosphate and 3′-hydroxyl ends.
Restriction system recognizing palindromic sequence typically cut within the recognition site not only produces single strand protruded ends but also produce blunt ends with no protruding bases. When these enzymes do not recognize palindromic sequences typically cut DNA a number of bases away from the recognition site to leave either 5′ or 3′-single-stranded protrusion.
Purification of Restriction Enzymes:
Purification of (Type II) enzymes involves analysis followed by high speed centrifugation. Further purification is achieved when the DNA binding protsins are bound to DNA cellulose and subsequently eluted with gradient of increasing concentration.
The eluted fractions are incubated with standard DNA such as plasmid or phage DNA, and then confirmed by gel electrophoresis for the determination of restriction digestion activity.
The contaminated enzymes are subjected to further purification which involves chromatography with DEAL sephadex, or other resins. It takes 3 to 4 days for the purification of 5,000 to 200,000 units of enzyme from 45 to 50 gm of bacteria. The dialized enzyme is stored at – 20°C by concentration for 10 to 100 units/µl.