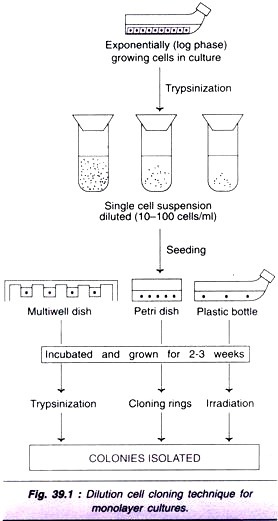
The following points highlight the three major causes of cancer. The causes are: 1. Radiant Energy 2. Chemical Compounds 3. Oncogenic Viruses.
Cause # 1. Radiant Energy:
(a) Ultraviolet rays, X-rays, and y-rays damage DNA in several ways.
(b) Ultraviolet radiation may cause pyrimidine dimers to form.
(c) Apurinic or apyrimidinic sites may form by elimination of corresponding bases. Single and double-strand breaks or cross- linking of strands may occur.
(d) The basic mechanism of carcinogenicity with radiant energy is to cause damage to DNA.
(e) Free radicals are formed in tissues by X- rays and y-rays. The resultant OH, superoxide, and other radicals can interact with DNA and other macromolecules, leading to molecular damage and thereby probably contributing to carcinogenic effects of radiant energy.
Cause # 2. Chemical Compounds:
(a) It has been estimated that environmental factors, principally chemicals, can cause up to 80 per cent of human cancers.
(b) Exposure to such compounds can occur because of a person’s occupation (e.g., benzene, asbestos); diet (e.g., aflatoxin B1 which is produced by the mold Aspergillus flavus and sometimes found as a contaminant of peanuts and other foodstuffs); Life style (e.g., cigarette smoking); or in other ways (e.g., certain therapeutic drugs can be carcinogenic).
(c) The carcinogenic substances may be both organic and inorganic molecules.
The following table shows some chemical compounds which may cause cancer:
Action:
The organic carcinogens, such as nitrogen mustard and β-propiolactone, interact directly with target molecules (direct carcinogens), but others require prior metabolism to become carcinogenic (Pro-carcinogens). The process by which one or more enzyme-catalyzed reactions convert pro-carcinogens to active carcinogens is called metabolic activation.
Any intermediate compound formed is proximate carcinogens, and the final compound that reacts with cellular components (e.g., DNA) is the ultimate carcinogen. Pro-carcinogen → Proximate carcinogen A → Proximate carcinogen B → Ultimate carcinogen.
(b) The ultimate carcinogen is highly reactive and is usually electrophile (molecule deficient in electron). This readily attacks nucleophilic (electron-rich) group in DNA, RNA, and protein.
Mono-oxygenases and Transferases:
(a) Mono oxygenases and transferases cause the metabolism of pro-carcinogens and other xenobiotics.
(b) The heme-containing mono-oxygenases of the cytochrome P-450 type located in the endoplasmic reticulum are mainly responsible for the metabolic activation of pro-carcinogens.
(c) The mono-oxygenases catalyze the hydroxylation of various pro-carcinogens and other xenobiotics using molecular oxygen as the source of oxygen and NADPH as a reducing source.
R − H + O2 + NADPH + H → R 0H + H2O+ NADP+
(d) At least 6 or many more such monoxygenases are present in the endoplasmic reticulum of human liver.
(e) The specific mono-oxygenase responsible for the metabolism of polycyclic aromatic hydrocarbons is named cytochrome P-448 or aromatic hydrocarbon hydroxylase. The reactions catalyzed by these mono-oxygenases are called phase I reactions of xenobiotic metabolism.
(f) In phase 2 reactions of xenobiotic metabolism, the hydroxylated xenobiotics are conjugated with various moieties (e.g., Glucuronate, sulfate, acetate, glutathione). These reactions usually detoxify the reactivity of the compounds involved and make them ready for excretion, mainly in the urine.
(g) In some cases, conjugation actually increases the biologic activity or chemical reactivity of a molecule. The enzymes catalysing the above conjugation reactions are usually cytosolic in location, although some are also present in the endoplasmic reticulum. The various glutathione transferases use glutathione transferases utilizing glutathione itself as the donor.
Factors affecting enzymes metabolizing xenobiotics:
The following factors affect the activities of the enzymes metabolizing xenobiotics:
(i) The activities of these enzymes may differ substantially among species.
(ii) Significant differences are found in enzyme activities among individuals, many of which are due to genetic factors.
(iii) The activities of some of the enzymes vary according to age and sex.
(iv) Intake of phenobarbital, PCBS, or certain hydrocarbons can also increase the activities of many enzymes by a process known as enzyme induction. Hydrocarbon inhalation from cigarette smoking during pregnancy induces the activity of cytochrome P-448 in the placenta altering the amounts of certain metabolites of hydrocarbons to which the fetus is exposed.
(v) The metabolites of certain drugs can inhibit the activities of xenobiotic-metabolizing enzymes.
Mutagens:
(a) Most of the chemical carcinogens are mutagens.
(b) The use of bacteria is mutagenicity tests creates a problem that they do not contain the spectrum of mono-oxygenases found in higher animals.
Initiation and Promotion:
(a) The stage of carcinogenesis of the skin of experimental mice caused by the application of benzo [a] pyrene is called initiation and this stage is rapid and irreversible. It is supposed to involve an irreversible modification of DNA resulting in one or more mutations. Benzo [a] pyrene is thus called an initiating agent.
(b) The second stage of carcinogenesis, resulting from the application of croton oil, is called promotion and croton oil is, therefore, a promoter. Promoters can cause initiation.
(c) Most carcinogens can act as both initiating and promoting agents.
(d) A good number of compounds including phenobarbital and saccharin can act as pro inoters in different organs.
(e) The active agent of croton oil is a mixture of phorbol esters. The most active phorbol ester is 12-0-tetra-de-canoyl-phorbol-13-acetate (TPA) which has numerous effects.
(f) Protein Kinase C can act as a receptor for TPA. The enzyme being stimulated by interaction with TPA may result in the phosphorylation of a number of membrane proteins resulting in the effects on transport and other functions.
Role of DNA:
DNA is the premier target molecule in carcinogenesis which is being established by the following facts:
(i) Cancer cells beget cancer cells, i.e., the required changes responsible for cancer are transmitted from mother to daughter cells. This is consistent with the behaviour of DNA.
(ii) DNA is damaged by both irradiation and chemical carcinogens which are capable of causing mutations in DNA.
(iii) Many tumor cells exhibit abnormal chromosomes.
(iv) Transfection experiments show that purified DNA (oncogenes) from cancer cells can transform normal cells into cancer cells. Epigenetic factors may also play a role in carcinogenesis.
Cause # 3. Oncogenic Viruses:
(i) Oncogenic viruses contain either DNA or RNA as their genome.
(ii) Polyomavirus and SV 40 viruses have played an important role in the development of current ideas about viral oncogenesis. Both of them are small and their circular genomes code for only about 5-6 proteins. Under certain circumstances, appropriate cells being infected with these viruses can result in malignant transformation. Specific viral proteins are involved too.
(iii) In case of SV 40, these proteins (often called antigens) are known as T and t, and in case of polyomavirus, they are known as T, mid-T, and t (T refers to the first of these proteins detected in a tumor).
(iv) The T antigens are to bind tightly to DNA and cause alteration in gene expression. These proteins show cooperative effects, suggesting that alteration of more than one reaction or process is required for transformation.
(v) Transformation of certain animal cells are caused by some types of adenovirus.
(vi) Epstein-Barr virus is associated with Burkitt’s Lymphoma and nasopharyngeal carcinoma in humans.
(vii) Herpes Simplex virus is associated with cancer of the cervix, and hepatitis B virus is also associated with some cases of liver cancer in humans.
Transformation:
The cultured cells undergo malignant transformation when they are infected with certain oncogenic viruses. These changes affect cell shape, motility, growth, and a number of biochemical processes. They reflect the conversion from the normal to the malignant state.
Acquisition by cells of the changes collectively known as transformation does not mean that such cells will display the same biologic properties as tumor cells in vivo; cells must yield tumors when injected into a suitable host animal.
Oncogenes:
Oncogenes are genes capable of causing cancer. These were first recognised as unique genes of tumor-causing viruses that are responsible for the process of transformation (viral oncogenes).
Oncogenes of Rous Sarcoma Virus:
(i) The genome of this retrovirus contains four genes named gag, pol, env, and src.
(ii) The gag gene codes for group-specific antigens of the virus, pol for the reverse transcriptase that characterizes retroviruses, and env for certain glycoproteins of the viral envelope. A protein-tyrosine kinase was shown to be the product of src (i.e., the sarcoma-causing gene) that is responsible for transformation.
(iii) Certain glycolytic enzymes become target proteins for the src protein-tyrosine kinase. This shows that transformed cells often show increased rates of glycolysis. The product of src may also catalyze phosphorylation of phosphatidylinositol to phosphatidylinositol mono- and bi-phosphate.
(iv) When phosphatidylinositol 4, 5-bi-phosphate is hydrolyzed by the action of phospholipase C, 2 second messengers are released: inositol triphosphate and diacylglycerol. The first compound mediates release of Ca++ from intracellular sites of storage (e.g., the endoplasmic reticulum).
(v) Diacylglycerol stimulates the activity of the plasma membrane-bound proteins kinase C which in turn phosphorylase a number of proteins, some of which may be components of iron pumps.
(vi) Mild alkalinization of the cell brought about by activation of an Na+/H+ anti-port system can play a role in stimulating mitosis.
The product of src may, therefore, affect a large number of cellular processes by its ability to phosphorylate various target proteins and enzymes and by stimulating the pathway of synthesis of the polyphosphoinositides.
Oncogenes of other Retroviruses:
(i) About 20 oncogenes of other retroviruses have been identified. Almost half of the products are protein kinases, mostly of the tyrosine type.
(ii) Some of these encode protein kinases, the remainder encode various other proteins with interesting biologic activities.
(iii) The product of the ras oncogene of murine sarcoma viruses binds GTP, has GTPase activity, and is related to the proteins that regulate the activity of the important plasma membrane enzyme, adenylate cyclase.
Mechanism by which proto-oncogenes become Oncogenes:
A. Promoter Insertion:
(i) When the particular viruses infect cells, a DNA copy (cDNA) of their RNA genome is synthesized by reverse transcriptase, and the cDNA is integrated into the host genome. The integrated double-stranded cDNA is called a Provirus.
(ii) Following infection of chicken B lymphocytes by certain avian leukemia viruses, their proviruses become integrated near the myc gene. The myc gene is activated by an upstream, adjacent viral long terminal repeat acting as a promoter, resulting in transcription of both the corresponding myc mRNA and translation of its product in such cells.
B. Enhancer Insertion:
(i) In certain cases, the provirus is inserted downstream from the myc gene or upstream from it but oriented in the reverse direction, the myc gene never become activated. Such activation cannot be due to promoter insertion.
(ii) Enhancer sequences present in the long terminal repeat sequences of the retroviruses.
(iii) The above two mechanisms—promoter and enhancer insertion—commonly operate in viral oncogenesis.
C. Chromosomal Translocations:
(i) Many tumor cells show chromosomal abnormalities. Translocation is a type of chromosomal change seen in cancer cells.
(ii) A piece of one chromosome being split off joins to another chromosome and if the second chromosome donates material to the first, the translocation is said to be reciprocal.
(iii) A number of tumor cells show characteristic translocations. One important translocation is the Philadelphia chromosome occurring in chronic granulocytic leukemia.
(iv) Burkitt’s Lymphoma is a fast-growing cancer of human B Lymphocytes.
(v) Synthesis of greatly increased amounts of the DNA-binding protein coded for by the myc gene acts to “drive” or “force” the cell towards becoming malignant by an effect on the regulation of mitosis.
D. Gene Amplification:
(i) One method is shown in respect of gene amplification in tumors by administration of the anticancer drug methotrexate, an inhibitor of the enzyme dihydrofolate reductase. Tumor cells can become resistant to the action of this drug.
(ii) Certain cellular oncogenes can also be amplified and are thus activated.
(iii) Increased amounts of the products of certain oncogenes produced by gene amplification may play a role in the progression of tumor cells to a more malignant state.
E. Single-point Mutation:
(i) The product of murine retroviruses, a protein of MW 21000 is related to the G proteins that modulate the activity of adenylate cyclase and thus play a key role in cellular responses to many hormones and drugs.
(ii) The lower activity of GTPase can result in chronic stimulation of the activity of adenylate cyclase which normally is diminished when GDP is formed from GTP. The resulting stimulation of the activity of adenylate cyclase can result in a number of effects on cellular metabolism exerted by the increased amount of cAMP affecting the activities of various cAMP-dependent protein kinases.
General Comments, on Activation of Oncogenes:
(i) Increased amounts of the product of an oncogene may be sufficient to push a cell towards becoming malignant.
(ii) The presence of a structurally abnormal key regulatory protein in a cell may also be sufficient to tip the scales towards cancer.
(iii) Oncogenes have been isolated from only about 15 per cent of human tumors.
(iv) Recent work has shown that activation of C-ras in rat mammary cancers induced by nitrosomethylurea was apparently due to a specific G → A transition type of mutation, demonstrating that oncogenes are probably involved in chemical carcinogenesis.
(v) Further research is essential to examine the possible involvement of oncogenes in the phenomena of initiation, promotion, tumor progression, and metastasis.
Mechanisms of Action of Oncogenes:
(i) They may act on key intracellular pathways involved in growth control uncoupling them from the need for an exogenous stimulus.
(ii) The products of oncogenes may also imitate the action of a polypeptide growth factor.
(iii) The products may also imitate an occupied receptor for a growth factor.
Polypeptide Growth Factors:
(i) The growth factors affect many different types of cells, e.g., cells from the blood, nervous system, mesenchymal tissues, and epithelial tissues.
(ii) They exert a mitogenic response on their target cells.
(iii) Platelet-derived growth factor (PDGF) released from the alpha granules of platelets plays a role in normal wound healing. Various growth factors play key roles in regulating differentiation of stem cells to form various types of mature hematopoietic cells. Growth inhibitory factors also exist. Thus, chronic exposure to increased amounts of a growth inhibitory factor can alter the balance of cellular growth.
Endocrine, Paracrine and Autocrine Actions of Growth Factors:
Growth factors may act in the following ways:
(i) Their effects may be endocrine, like hormones, they may be synthesized elsewhere in the body and may pass in the circulation to their target cells.
(ii) They may be synthesized in certain cells and secreted from them to affect neighbouring cells. The cells that synthesize the growth factor are not themselves affected because they lack suitable receptors. This mode of action is called paracrine.
(iii) Some growth factors can affect the cells that synthesize them. This third mode of action is called autocrine.