The following points highlight the nine main biochemical mechanisms of action of growth factors of cancer cells. The biochemical mechanisms are: 1. Progression of Tumors 2. Drugs used in Cancer Chemotherapy 3. P-Glycoprotein Plays an Important Role in Multidrug Resistance in Cancer Chemotherapy 4. Metastasis 5. Isolation of a Number of Genes Involved in the Increased Susceptibility to Cancer and Others.
Biochemical Mechanisms of Action of Growth Factors:
- Progression of Tumors
- Drugs used in Cancer Chemotherapy
- P-Glycoprotein Plays an Important Role in Multidrug Resistance in Cancer Chemotherapy
- Metastasis
- Isolation of a Number of Genes Involved in the Increased Susceptibility to Cancer
- P53 Tumor Suppressor Gene Acts as a Guardian of the Genome
- Mutations in P53 occur in many Human Tumors
- Malignancy of Tumor Cells Tends to Progress
- Telomerase is Active in many Cancer Cells
Biochemical Mechanism # 1.
Progression of Tumors:
(i) A cell once becomes a tumor cell, tendency for malignancy is increased. This is signified by increasing rates of growth, and increasing tendency to invade and metastasize.
(ii) Cells having faster rates of growth will have a selective advantage.
(iii) The biochemical affairs of highly malignant cells may be very different from that of normal cells. Some changes are secondary to rapid growth rates and others are due to chromosomal instability.
(iv) The fast-growing cells tend to increase the anabolic processes involved in growth (e.g., DNA and RNA synthesis), cut down on catabolic functions (e.g., catabolism of pyrimidine’s), and dispense with the differentiated functions shown by their normal ancestors.
In fact, they are mostly concentrated upon growth. They also show biochemical changes that reflect altered gene regulation, such as the synthesis of certain fetal proteins.
Biochemical Mechanism # 2.
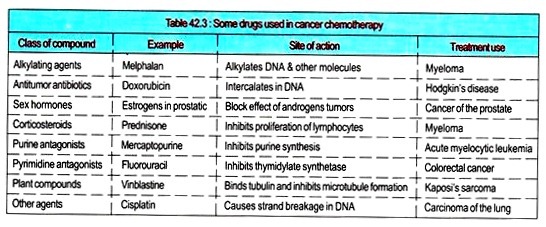
Drugs used in Cancer Chemotherapy:
i. The patients with cancer are well treated by surgery, radiotherapy, and chemotherapeutic agents. It is always advisable to see that the drugs (natural products or synthetic) that kill cancer cells effectively must not toxic to normal cells.
ii. Many of the agents are used because they inhibit DNA synthesis. For this reason, they cause damage to normal tissues whose cells divide continuously (e.g., the gut and bone marrow).
iii. The growth fraction (the cells of tumor that are constantly in cycle) is an important factor in cancer chemotherapy; tumors with a high growth fraction are usually more responsive to cancer chemotherapy. It is wise to classify anticancer drugs as cell cycle specific (CCS) or cell cycle nonspecific (CCNS).
iv. CCS agents are further subdivided into phase-specific agents (e.g., agents acting on the S phase of the cycle [e.g., cytarabine] or on the G2-M phase [e.g., bleomycin].
v. In all cancer therapy, it is vital to diagnose and treat the tumor early; otherwise, the tumor burden may be too great for therapy to be successful. During therapy, it is wise to eliminate all clonogenic cells (“tumor stem cells”).
vi. Combination chemotherapy (combining three or four agents) is successful in some cancers because the drugs may act synergistically, the onset of resistance may be delayed, and toxicity is often less.
Biochemical Mechanism # 3.
P-Glycoprotein Plays an Important Role in Multidrug Resistance in Cancer Chemotherapy:
i. A particular drug is never effective against the target tumor cells.
ii. The acquired resistance of cancer cells to chemotherapeutic agents is to reflect their high mutation rates. Resistance is not only to the drug but also to other structurally unrelated anticancer agents, e.g., if resistance to methotrexate develops, the tumor cells may also be found to be resistant to antitumor antibiotics such as doxorubicin and to plant compounds such as vincristine. This is known as multi drag resistance which is important as its development is frequently the cause of failure of cancer chemotherapy, a common event during the treatment of cancer.
iii. The protein, P-glvcoprotem (P = permeability) acts as an energy-dependent pump, ejecting a wide variety of anticancer drugs from cancer cells and thus diminishing their efficacy.
iv. P-glycoprotein has been the subject of much study because of its therapeutic importance. In cultured cells, gene amplification and point mutations have been found in resistant cancer cells showing elevated amounts of P-glycoprotein.
v. P-glycoprotein has physiologic functions since it is present in normal organs, such as kidney and gut; it may play a role in the excretion of potentially toxic compounds from cells of these organs.
vi. P-glycoprotein can be inhibited by chemo-sensitizers such as verapamil or cyclosporine.
Biochemical Mechanism # 4.
Metastasis:
Metastasis is a complex process to analyse in humans. It is the spread of cancer cells from a primary site of origin to other tissues where they grow as secondary tumors, it is the major problem presented by the disease. Biochemical knowledge regarding this is quite restricted.
Much attention has been put to the comparisons of the biochemistry of the surfaces of normal and malignant cells.
At present, metastasis has been studied by considerable research work on developing suitable animal model systems. Much studies are being carried out to identify the possible roles of certain proteases (e.g., type 4 collagenase) and of certain glycoproteins and glycosphingolipids of the cell surface is the process of metastasis.
For instance, it may be possible that changes in the oligosaccharide chains of cell glycoproteins may be effective in permitting metastasis to occur. The biochemical mechanisms involved in metastasis may provide a basis for the rational development of more effective anticancer therapies.
Biochemical Mechanism # 5.
Isolation of a Number of Genes Involved in the Increased Susceptibility to Cancer:
(a) Much progress has been made in isolating genes that increase susceptibility to cancer. Recently, BRCA1, a gene that influences susceptibility to early-onset breast and ovarian cancer has been isolated.
(b) BRCA1 is a protein of 1863 amino acids containing a zinc finger domain in its amino terminal region and is a transcription factor.
(c) About 12 other genes associated with familial types of cancer have been identified to specific chromosomal locations.
(d) The second gene of these 12 BRCA2 is associated with increased susceptibility to breast cancer only and mapped to chromosome 13q12-13.
(e) The further study of these genes will facilitate early diagnosis of different types of familial cancer and will provide to know the mechanisms of development of cancer in various tissues.
Biochemical Mechanism # 6.
P53 Tumor Suppressor Gene Acts as a Guardian of the Genome:
(a) P53 is nuclear phosphoprotein of molecular mass 53KDA.
(b) It is located on short arm of chromosome 17.
(c) It binds specific sequences in dsDNA.
(d) It acts as a transcriptional regulator regulating genes that participate in cell division.
(e) It binds various viral proteins forming inactive oligomeric complexes.
(f) It acts as G, checkpoint control for DNA damage. If DNA is excessively damaged, the activity of P53 is increased resulting in inhibition of cell division and allowing time for repairing.
(f) It participates in the initiation of apoptosis which is defined as programmed cell death occurring in many physiologic and pathologic conditions.
(g) It hastens the death of potentially dangerous cells which have the potential to become malignant. In such affair, P53 has been referred to as a “guardian of the tissues”.
Biochemical Mechanism # 7.
Mutations in P53 occur in many Human Tumors:
(a) Mutations in the P53 gene are the most common genetic change in human cancer and are often in colon, breast, and lung cancers.
(b) At least 100 different mutations have been detected in this gene.
(c) The mutations are found at highly conserved codons.
(d) The mutational spectrum differs in different cancers.
(e) Transitions occurring at CpG di-nucleotides (a hot spot) are frequent in neoplasms of the colon, brain, and lymphoid tissue.
(f) Transversions are frequent in lung cancer and liver cancer and are found mainly at one specific site in certain hepatocellular carcinomas occurring in areas where exposure to aflatoxin B, and hepatitis B virus.
Biochemical Mechanism # 8.
Malignancy of Tumor Cells Tends to Progress:
i. When a cell becomes a tumor cell, the composition and behaviour of the cell do not remain static. Rather, tendency for malignancy is increased. This is manifested by increasing rates of growth, and an increasing tendency to invade and metastasize.
ii. The mutations in DNA repair genes are found in this phenomenon creating a mutator phenotype. The activation of additional oncogenes occurs. Cells are found with faster rates of growth.
iii. The biochemical profile of highly malignant cells may be very different from that of normal cells. Many changes in enzyme profile also occur.
iv. The fast growing cells tend to maximize the anabolic processes involved in growth, cut down on catabolic functions. Briefly speaking, they are concentrating mostly upon growth.
v. They also show biochemical changes that reflect altered gene regulation, such as the synthesis of certain fetal proteins and the manufacture of hormones.
Biochemical Mechanism # 9.
Telomerase is Active in many Cancer Cells:
i. Telomeres are compound of DNA and proteins that are located at the ends of chromosomes of lower and higher eukaryotes. They confer stability on the ends of chromosomes.
ii. In humans, telomeres consist of 1000 or more arrays of TTAGGG repeats at the terminals of the 3′ ending strands. These repeats are maintained in germ-line cells by ” the action of telomerase.
iii. Telomeres are observed to shorten during sequence of primary cultures of cells and cultured primary cells are found to lack telomerase activity.
iv. Telomeres as well as telomerase are proved to be useful targets for anticancer therapy. A good number of normal cells contain telomerase activity. The enzyme telomerase is active in normal or malignant cells with a high proliferation rate. There is evidence that telomerase is active in some tumors and not others. Measurement of its activity may help in the staging of certain tumors.