In this article we will discuss about:- 1. Habit and Habitat of Selaginella 2. Salient Features of Selaginella 3. Structure 4. Reproduction.
Habit and Habitat of Selaginella:
Selaginella shows considerable variation in size, symmetry and morphology. Mostly they are herbaceous perennials, however, a few are annuals (Selaginella pygmaea).
Majority are dorsiventral, prostrate and creeping (Fig. 7.45) on the surface (e.g., Selaginella kraussiana; S. pellidissima; S. chrysocaulis), some are radial and grow erect (e.g., S. rupestris; S. viridangula; S. selaginoides) and few are scandent (e.g., 5. willdenovii; 5. adunca) and climbers (5. alligans).
Most of the species of Selaginella grow on the ground in humid, shady habitats. A few species are xerophytic and grow in arid conditions on exposed rock surfaces (5. pilifera; S. lepidophylla). They have the ability to survive desiccation.
Resurrection Plant:
Some xerophytic species (e.g., S. lepidophylla, S. pilifera, 5. bryopteris) rolls up into a compact ball of seemingly dead leaves and stems during dry seasons, but unrolls to carry on normal activities when put in water. Such plants are commonly known as the “resurrection plants” and are often sold as curiosities.
Salient Features of Selaginella:
i. The sporophyte is herbaceous and the shoot is dorsiventral and radial and creeping or erect.
ii. The leaves are small (microphyllous) and a ligule is present at the base of each leaf and sporophyll.
iii. Rhizophore is (a leafless structure where from roots arises) present in some species.
iv. Sporophylls are usually aggregated into strobili at the apices of the branch, hetero- sporous.
v. Heterothallic (dioecious) gametophytic prothalli.
vi. Antherozoids are biciliate.
Structure of Selaginella:
The Sporophyte:
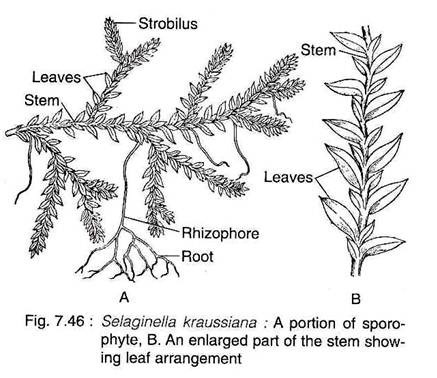
The plant body of Selaginella is differentiated into well developed roots, stem and leaves.
Besides, some species also have rhizophore (Fig.7.46A).
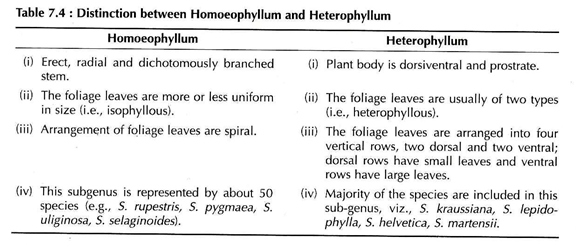
Hieronymus (1902) divided the genus Selaginella into two sub-genera viz., Homoeophyllum and Heterophyllum on the basis of general structure of the plant body (Table 7.4).
Stem:
The stem is generally erect and radially constructed in the subgenus Homoeophyllum. The species belonging to the subgenus Heterophyllum are prostrate and dorsiventral. The branching is dichotomous in the member of Homoeophyllum and somewhat lateral in Heterophyllum.
The anatomy of the mature stem is very distinct and is differentiated into an outer epidermis, middle cortex and centrally located stele (Fig. 7.47). The outer cell walls of the epidermis are cutinised. It is devoid of stomata and hairs. In many species there are several layers of thick- walled cells (hypodermis) beneath the epidermis, which merge gradually with thin-walled chlorophyllous cells of the cortex.
The cortex is usually made up of compactly arranged angular parenchymatous cells without intercellular spaces. The most part of the cortex of xerophytic species viz., S. lepidophylla and 5. rupestris, is comprised of thick-walled sclerenchymatous cells.
There is much variation in the vascular cylinder among the different species of Selaginella. The number of stele varies from species to species and sometimes even in the same species. This is due to the dissection of the main stele. Young plants invariably show monostelic configuration.
In most species the creeping stem or prostrate rhizome is protostelic. Species with radial symmetry may have a single, cylindrical proto- stele in the stem (monostelic e.g., S. spinulosa, S. flabellata), whereas dorsiventral species may have two (e.g., distelic e.g., S. kraussiana) or more vascular strands (12-16 steles) (polystelic e.g., S. laevigata) that are either circular or ribbon-shaped.
The number of steles may vary in different parts of the same plant. In S. braunii, the creeping prostrate axis is bistelic, while the erect branches are monostelic. In S. lyalli prostrate branches are bistelic, whereas erect branches are polystelic.
Each stele is surrounded by a single-layered pericycle. The stele is set off from the cortex by a few radially elongated endodermal cells designated as trabeculae. They have the characteristic casparian bands on their radial walls. Thus, the central stele is separated from the cortex by large air spaces.
Regardless of the stelar organisation, the primary xylem is exarch in development and the metaxylem consists primarily of tracheids with scalariform pittings.
The phloem of Selaginella consists of sieve cells and parenchyma. In some species (S. densa, S. rupestris, S. oregana) the xylem possesses true vessels with transverse perforation plates.
Rhizophores:
(Greek rhiza = root; phora = bearer).
In many species of Selaginella, peculiar leafless, prop-like cylindrical, structures, originate from the stem at the point of branching. These grow downwards into the surface and form many adventitious roots at their free ends. They are known as rhizophores.
A T.S. of the rhizophore shows features very much similar to the root, however, with some mild variations (Fig. 7.48). The epidermis is single-layered and highly cutinised. The cortex is extensive and may be differentiated into an inner thin-walled parenchymatous and outer thick-walled sclerenchymatous zone (hypo- dermis).
The stele is protostelic and surrounded by a layer of endodermis. It shows variations in its form and arrangement of protoxylem in different species of Selaginella. In S. martensii, the xylem is monarch and exarch.
In S. atroviridis the metaxylem is crescent-shaped and the protoxylem occurs in the form of a few groups on the concave adaxial side. In S. kraussiana, the stele is centroxylic where a single concentric stele with one protoxylem lying in the centre.
Morphological Nature of Rhizophore:
The morphological nature of rhizophore is controversial because of its unusual position and structure. It has been interpretated by various plant scientists as root, stem or an organ sui generis (i.e., an organ, neither a stem nor a root).
I. Similarities with Root:
i. Rhizophores are positively geotropic in nature.
ii. It does not bear leaves.
iii. Monarch xylem like that of root.
iv. Presence of root cap in some species, e.g., S. densa, S. kraussiana, S. martansii, S. wallacei (Webster and Steeves, 1967).
v. Transport of auxin in rhizophore is acropetal which is similar to root (Wochok and Sensex, 1974).
II. Similarities with Stem:
i. Exogenous in origin like stem.
ii. Absence of root caps and root hairs.
iii. Originate due to the activities of meristems which are present between the two branches of the stem. This meristem has been termed as angle meristem which is basically an embryonic shoot (Curick, 1959).
iv. Production of roots endogenously from the tip.
v. Under experimental conditions the rhizophore can be transformed into a leafy short (Foster and Cifford, 1959).
Hence Bower (1908, 1935) and Goebel (1905) suggested that rhizophore is neither a root nor a stem, but an organ sui generis. According to Schoute (1938) it is a specialised stem behaves like root.
However, recent biochemical studies of protein from stem, leaf, root and rhizophore revealed that the polypeptides of the rhizophore more closely resemble those of the stem rather than subterranean roots. The above-mentioned features are not for a typical root, moreover, they produce roots endogenously. Therefore, these outgrowths are called rhizophores (Gr. rhiza = root; phora = bearer).
Root:
The roots of the young sporophyte are ephemeral, but the roots of the mature sporophyte are adventitious in nature and dichotomously branched. Generally they arise at points of branching of the stem from a meristem, termed as angle meristem.
A T.S. of a root shows a very simple arrangement with a centrally located protostele covered by parenchymatous cortex and bounded externally by cuticularised epidermis. In some species, the outer layers of cortex become sclerenchymatous and form hypodermis. The stele consists of a small, exarch and monarch xylem.
Leaf:
The leaves of all species are microphyllous, sessile and simple, attaining a length of a few millimeters. The leaves may be ovate, lanceolate or orbicular in shape with one vein running nearly the entire length of the leaf. All the leaves are of same type and the arrangement is spiral in subgenus Homoeophyllum.
In the sub-genus Heterophyllum, the leaves are of two types; small leaves and large leaves, that are arranged in four rows along the stem. There are two rows of small leaves on the dorsal side of the stem and two rows of larger leaves on the ventral side or in a lateral position (Fig. 7.46B).
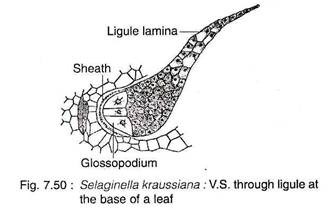
A small, membranous, tongue-like structure, ligule (latin ligula = a small tongue), is located at the base of each vegetative, leaf and sporophyll (Fig. 7.51). The ligule is found on the ventral (upper) surface of the leaf.
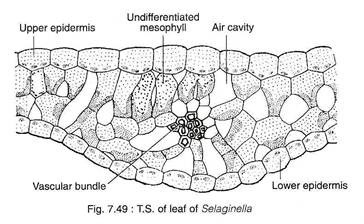
A T.S. of a leaf shows two epidermal layers, mesophyll tissue, stele and stomata (Fig. 7.49). The cells of upper and lower epidermal layers may be similar in most of the species (e.g., 5. rupestris). However, they may be somewhat different in some species (e.g., 5. martensii) where the upper epidermis consists of conical cells, but the cells of the lower epidermis are smaller.
Some species develops bristles or short hair-like structures extending out from the epidermis. The mesophyll consists of loosely arranged spongy parenchyma with intercellular spaces.
The mesophyll tissue may be differentiated into a distinct palisade and spongy parenchyma layers (e.g., S. lyalli, S. concinna) or the entire mesophyll may look like a reticulation lacunate parenchyma cells. A mesophyll cell contains 1-8 cup-shaped chloroplasts with many pyrenoid-like cells.
The stomata are present generally on the abaxial (lower) surface, although in certain species they are present on both the surfaces (amphistomatic). A single vascular bundle composed of central xylem surrounded by phloem is present at the centre. The bundle is bounded by a distinct bundle sheath.
Ligule:
The ligule has a basal cup-shaped sheath with often casparian strips (e.g., S. kraussiana) and an adjacent hemispherical glossopodium (Greek glossa ‘tongue’ and podiun ‘foot’) made up of a group of large, vacuolated cells (Fig. 7.50). The remaining cells are more or less cubical in shape. The terminal end of ligule is thin and single-layered. The basal portion of the ligule is somewhat sunken into the tissues of the leaf.
The ligule acts as a water-absorbing structure which keeps the young leaf primordium and sporangium of sporophyll moist during their early development. The ligule in some species, during early development, secretes a mucilagenous substance composed of carbohydrates and proteins that keep the apical meristem moist and prevent them from desiccation (e.g., S. wallacei, S. kraussiana).
Reproduction in Selaginella:
The sporophyte of Selaginalla reproduces by vegetative and sexual methods.
i. Vegetative Reproduction:
The vegetative reproduction in Selaginella takes place by tubers (e.g., S. chrysocaulis), bulbils (S. chrysorhizos), dormant buds (S. chrysocaulis) and fragmentation (S. rupestris).
Bulbils and dormant buds are produced in aerial branches, while tubers may be aerial or underground. In favourable condition they germinate to produce new sporophytic plants. In S. rupestris, the trailing branches of the stem develop adventitious branches, that separate from the parent plant and grow into new sporophyte.
ii. Reproduction by Spores:
Numerous haploid spores are produced in the sporangium. The sporangium are located in the sporophylls and the sporophylls are compactly arranged to form cones or strobili.
Strobilus:
All the species of Selaginella forms strobili or cones. Generally strobili occur terminally on side branches, but in some species (e.g., S. patula and S. cuspidata), the apical meristem of the cone may continue meristematic activity producing foliage (vegetative) leaves and, therefore, produces a shoot with sporophylls (sporangium bearing leaves) and foliage leaves in alternate segments along the axis.
Selaginella is heterosporous and, therefore, sporangia are of two types viz., microsporangia and megasporangia. The sporophylls associated with these two types of sporangia are designated as microsporophylls and megasporophylls respectively.
There is variation in distribution of sporangia within the strobili of different species. Strobili either consists entirely of microsporangia or of megasporangia (S. gracilis, S. atroviridis). However, the mixed condition is more common (Fig. 7.51).
The lower portion of a strobilus consists of megasporangia and the upper portion of microsporangia (S. helvetica, S. rupestris, S. selaginoides) or the two types of sporangia may be mixed indiscriminately.
In some species one side of strobilus bears microsporophylls and other side megasporophylls (e.g., S. inaequalifolia, S. oregana). In some species, only one megasporangium is present at the base of each strobilus, while the rest are microsporangia (e.g., S. kraussiana).
Sporangium:
The mature sporangia are stalked with two- layered jacket (Fig. 7.52A, B). The microsporangia are slightly elongated and reddish to bright orange in colour. Megasporangia are larger than microsporangia and are frequently lobed. The megasporangia are whitish-yellow or light orange in colour.
Development of Sporangium:
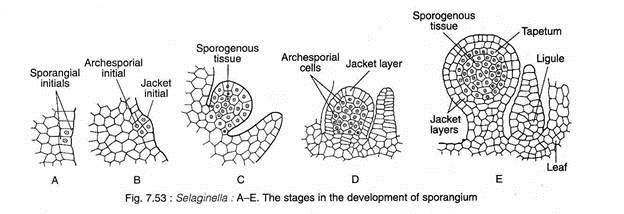
Initially the development of micro- and megasporangia are similar. The sporangial development is of eusporangiate type i.e., the sporangium develops from a group of initial cells (Fig. 7.53A-E).
The site of sporangial initiation (micro- or mega-sporangium) is in superficial cells of the axis, directly above the sporophyll, or in cells near the base of the sporophyll on the adaxial (upper) side.
Periclinal divisions in these initial cells produce two tier of cells: the outer jacket initials and the inner archesporial initials (Fig. 7.53A-B). A two-layered sporangial jacket is formed by repeated anticlinal and periclinal divisions of the jacket initial. The periclinal division of the archesporial cells produces an outer and inner layer of cells.
The outer layers of cells eventually develop into tapetum; the inner cells, by repeated divisions in various planes, produce sporogenous tissue (spore mother cells) (Fig. 7.53C-E). The sporangium at this stage consists of an immature sporangial wall of two layers, a short stalk and a conspicuous tapetal layer enclosing numerous sporogenous tissue. At this stage, microsporangia and megasporangia are indistinguishable.
Further Development of Sporangenous Tissue:
Differentiation of Micro- and Megasporangium:
Further development of sporogenous tissue gives rise to micro and megaspores. In micro- sporangium, about 80-90% of the sporogenous tissue undergoes meiosis and forms tetrads of haploid microspores. The microspores are more or less tetrahedral in shape. They are quite small in size (0.015 to 0.06 mm in diam.). The wall of the spore is divisible into outer thick and ornamented exine and inner thin intine.
In a potential megasporangium, only one spore mother cell becomes functional. The remaining non-functional megaspore mother cells develop large vacuoles and accumulate starch, while functional megaspore mother cell retains a dense cytoplasm which is rich in RNA and lacks starch.
The functional megaspore mother cell undergoes meiosis (reductional division) forming four megaspores. All the nonfunctional mother cells ultimately degenerate.
All the four megaspores develop from a megaspore mother cell may not be functional. Sometimes one (S. sulcata) or two (S. rupestris) megaspores are functional. Sometimes more than one megaspore mother cell in a megasporangium becomes functional resulting into 8, 12 or even more megaspores in a single megasporangium.
The resulting megaspores soon develop a thick-layered cell wall. The outer layer is thick and ornamented with spines or ridges known as exine (exospore). The inner layer is thin and termed as intine (endospore). In some species a third layer (mesospore) forms between exine and intine. The megaspores are much larger (diam. 1.5 to 5 mm) than the microspores.
Gametophyte:
The haploid spores germinate to form the endosporic gametophytes. The development of microspores and megaspores generally starts while they are still inside their respective sporangia. Therefore, the spores are shed at multicellular stage.
Male Gametophyte:
The microspore germinates to form the male gametophyte (Fig. 7.54A-I). The first unequal division (1-1) in a microspore results in the formation of a small lenticular prothallial cell (vegetative cell) and a large antheridial initial. The prothallial cell does not divide further. The antheridial initial divides (2-2) vertically forming two primary antheridial cells.
The upper two cells divide anticlinally (3-3) by means of a curved transverse wall forming four antheridial cells and the lower two cells do not divide constituting the first jacket cells of the antheridium. The antheridial cells nearer to the prothallial cell divide (4-4) periclinally by means of a curving vertical wall to form two small distal cells (2nd jacket cells) and two large inner cells (androgonial cells).
The other two antheridial cells divide (5-5) anticlinally to form two distal cells (3rd jacket cells and two proximal cells which again divide periclinally (6-6) by means of a curving vertical wall to form two outer (4th jacket) cells and two inner antrogonial cells.
Thus, at this stage, the male gametophyte has 13 cells (1 prothallial cell, 8 jacket cells and 4 androgonial cells) and male gametophyte is shed at this 13- celled stage (Fig. 7.54G, K).
After shedding, the four primary androgonial cells undergo several divisions forming 128 or 256 androcytes. Each androcyte gets metamorphosed into a biflagellate sperm (antherozoid) which on disintegration of the jacket cells and rupture of the spore wall along the triradiate ridge are liberated (as free-swimming sperm/antherozoid) (Fig. 7.54).
The antherozoids (sperms) are free-swimming type, very narrow and about 15-50 pm in diameter at about the middle of the sperm/antherozoid. Each flagellum is about 30 pm in length. The sperms of Selaginella are the smallest among vascular plants.
Since the whole process of development of male gametophyte takes place inside the microspore wall, the development is endosporic (where gametophyte develops inside the spore wall) and the gametophyte is an extremely reduced structure.
Female Gametophyte:
Like the male gametophyte, the megagametophyte (female gametophyte) development of Selaginella begins while the megaspores are still inside the megasporangium (Fig. 7.55A-E).
A conspicuous vacuole develops within the cytoplasm of the megaspore at a very early stage. Thus, the megaspore nucleus undergoes repeated nuclear divisions without cell wall formation (free nuclear division). As a result a thin layer of multinucleate cytoplasm is developed around the large vacuole (Fig. 7.55B, C).
The formation of cell walls around each nucleus starts initially at the apical end beneath the triradiate ridge and an apical patch of cells, two to three layered thick, is formed. In some species of Selaginella, the cell wall formation continues until the megagametophyte is entirely cellular.
In some species, e.g., S. kraussiana, the apical patch of cells is separated from the rest of the gametophyte by conspicuous arching diaphragm (wall). Ultimately the vacuole is obliterated by the formation of cells. The stage at which the megaspore is shed from the megasporangium varies from species to species.
In majority of the species, the final stages of female gametophyte development, and fertilisation and germination of young sporophyll take place while the megaspore with its enclosed megagametophyte rests on the substratum. The female gametophyte increases in size and exerts pressure on the megaspore wall.
This results in the splitting open the megaspore wall along the triradiate ridge. Tufts of rhizoids may develop from the exposed gametophytic tissue which play an important role in absorption of water and nutrients, also in anchorage.
However, in S. rupestris, the megaspores are not shed (retention of megaspore in megasporangium) and the development of female gametophyte and fertilisation take place in situ and the young sporophyte can be seen developing on the parent plant.
Development of Archegonium:
Archegonia originate in the apical region of the female gametophyte. Each archegonium develops from a single superficial cell (archegonial initial) which divides periclinally to form a primary cover cell and a central cell (Fig. 7.56A, B).
The primary cover cell follows two vertical divisions at right angle to each other to form four neck initials which again divide transversely to form eight neck cells, arranged in two tiers. The central cell divides periclinally to form an outer primary neck canal cell and an inner primary venter cell.
The primary neck cell does not divide further so that a neck canal cell is formed, while the primary venter cell divides transversely into a ventral canal cell and an egg (Fig. 7.56C-E). Thus, a single archegonium consists of eight neck cells arranged in two rows of four cells each, one neck canal cell, one ventral canal cell and an egg.
The venter, along with the inner tier of neck, lies embedded in the gametophytic tissue, while the terminal neck cells extend above the surface of the gametophytic tissue.
Fertilisation:
In majority of the species, fertilisation takes place after the megasporangium gets settled on the substratum, but in some cases (e.g., 5. rupestris), fertilisation occurs while the female gametophyte is still within the sporangium. Biflagellate sperms (haploid) are liberated, then they swim to the archegonia through a thin film of water and fertilise the egg (haploid) to form diploid zygote.
The Embryo (The New Sporophyte):
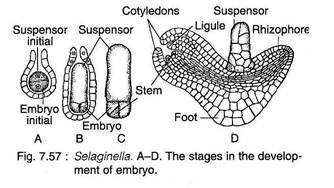
After fertilisation, the diploid sporophytic generation (i.e. zygote) is established. The first division of the zygote is generally transverse (Fig. 7.57A). The upper cell (epibasal) develops into one- or several-celled suspensor (that are toward the archegonial neck) and the lower cell (hypobasal) to the embryonic cell. The embryo is endoscopic (embryonic cell directed toward the base of the archegonium) in nature.
The embryonic cell divides by two vertical walls at right angles to each other resulting into a four-celled embryo (Fig. 7.57B). A short apical cell with three cutting faces is formed because of oblique divisions in one of the embryonic cells (Fig. 7.57C). At this stage, the embryo proper undergoes a 90-degree turn to its left.
The first pair of leaves are developed laterally on two sides of the shoot apex (Fig. 7.57D). Eventually a foot is formed on the lower side of the embryonic tissue and the primary root is developed between the suspensor and foot. The young sporophyte emerges from the gametophytic tissue through continued growth of the shoot and root.
How far Selaginella approaches seed habit?
Selaginella exhibits a significant approach towards seed habit because of the following notable features:
(i) It is a heterosporous pteridophyte.
(ii) In some species of Selaginella, viz., S. rupestris and S. monospora, the megaspore number is reduced to one.
(iii) In S. rupestris, the megaspore is retained within the megasporangium and the development of female gametophyte and subsequent fertilisation takes place in situ and even the young sporophyte can be seen developing on the parent plant.
(iv) Therefore, it becomes evident that the heterosporous vascular cryptogam, Selaginella, has considerably advanced towards seed habit in some species but its approach to the true seed is not complete due to the following reasons:
(a) The megasporangium wall is dehiscent and is not covered with the protective integuments,
(b) The retention of the megaspore permanently within the megasporangium has not become established,
(c) The absence of complete histological fusion between the megasporangium wall and the megaspore,
(d) The direct access of sperms to the egg,
(e) There is lack of resting period after the development of embryo.