The following points highlight the five main classes of chemical compounds in chromatin. The classes are: 1. Nucleic acids 2. Basic proteins or histones (protamines in sperm nuclei of some fishes and birds) 3. Acidic proteins (Non-Histones) 4. Lipoproteins 5. Associated inorganic molecules.
Chemical Compounds in Chromatin: Class # 1. Nucleic Acids:
The nucleic acids are of two types:
(i) Deoxyribonucleic acid (DNA), and
(ii) Ribonucleic acid (RNA).
DNA is present in the cells of all plants, animals, prokaryotes and in a number of viruses. DNA molecule appears to be the most stable substance that is present in the chromosome. It has also been reported in some cytoplasmic organelles like chloroplast, mitochondia and centrioles. The amount of DNA per set of chromosomes is constant for a particular species.
The DNA content of a diploid somatic cell (2n) is exactly double that of haploid cells (n). There is a close relationship between DNA content and chromosome size. It has been found that about 31cm of double helix DNA equals a pikogram (Ipg = 10-12g). In man a diploid cell contains about 5.6 pg of DNA which may correspond to about 174 cm of DNA.
RNA is found associated with chromosomes, in nucleolus, in the nuclear sap, ribosomes, chloroplast, mitochondria and some other cytoplasmic organelles.
According to function, RNAs are of two kinds:
(a) Genetic RNA:
In some viruses which contain RNA, RNA is the sole genetic material which carries all hereditary responsibilities.
(b) Non-genetic RNA:
In cellular organisms DNA is genetic material and RNA molecules do not carry out genetic functions. In these organisms different genetically controlled cellular functions are performed by different kinds of RNA called non-genetic RNAs.
Chemical Compounds in Chromatin: Class # 2. Histones or Basic Proteins:
Chromatin of all eukaryotic cells is a nucleoprotein complex in which DNA strand is associated with proteins. The associated proteins are of two kinds; basic proteins (histones) and acidic proteins (non-histones). Histones are small proteins that contain between 100 and 200 aminoacids of which 20 to 30% are lysine and arginine.
The histones bear positive charges which enable them to bind to DNA primarily by electrostatic attraction to the negatively charged phosphate groups in sugar-phosphate backbone of DNA. Histones also bind tightly to each other and both DNA-histone and histone-histone binding are important for chromatin structure.
The histones found in all eukaryotic chromosomes are five distinct types:
(i) H1 which is very rich in the basic amino acid lysine (mol. weight-approximately 21,500).
(ii) H2a and H2b which are lysine-rich (mol. wts. 14000 and 13775 respectively).
(iii) H3 and H4 which are rich in basic amino acid arginine (mol. wts. 15,320 and 11,280 respectively).
The five histones are present in molar ratios of approximately IH1: 2H2a : 2H2b : 2H3 : 2H4.
They are complexed with DNA to produce the basic structural subunits of chromatin, the nucleosome. The histones have been highly conserved during evolution and four of the five types of histones are similar in all higher eukaryotes. The sperms of a few eukaryotes are exceptions where the histones are replaced another class of small basic proteins called protamines.
The chromatin shows about equal amounts of histones and DNA.
Chemical Compounds in Chromatin: Class # 3. Non-Histones:
The non-histones or acidic proteins are found more or less firmly associated with DNA-histone complexes. In contrast to the histones, the molecules of non-histone proteins are numerous and heterogenous.
Their number in most of the organisms may be more than 100 and they may include the enzymes involved in the synthesis of DNA and RNA, i.e., DNA polymerase, RNA polymerase and protein molecules involved in the control of DNA and RNA synthesis.
The content of non-histones varies throughout the cell cycle and from one differentiated cell to another but the histones remain constant in both these instances. The amount of non-histone proteins generally equals the DNA-histones combined.
Inorganic Molecules:
Certain small inorganic molecules are also present in the chromosomes. These maintain the integrity of the chromosomes. Phosphorus, potassium, sodium, calcium and magnesium salts are very common in the nucleic material.
Lipoprotein Complexes:
Fats may also be found in the chromosomes, generally in association with proteins.
Components of Nucleic Acids:
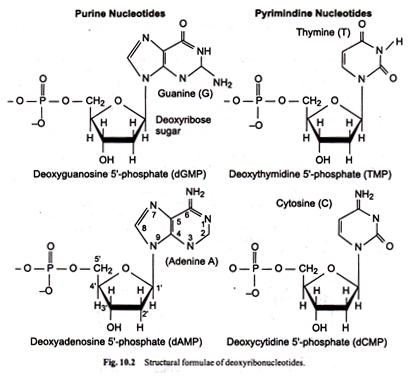
Nucleic acids, both DNA and RNA are linear polymeric macromolecules. The monomeric unit is termed nucleotide. It is deoxyribonucleotide or deoxyribotide in the case of DNA and ribonucleotide or ribotide in RNA.
A nucleotide consists of three components:
(i) a nitrogenous base, (ii) a pentose or 5 C-sugar, and (iii) a phosphate group. In the nucleotide, the combination of sugar with a nitrogenous base constitute a nucleoside. The hydrolysis of nucleic acids reveals a clear cut picture of their various components.
The various components of nucleic acids are discussed as follows:
Nitrogenous bases or Proteins:
The nitrogenous bases present in the nucleic acids are of two types:
1. Pyrimidines, and
2. Purine bases.
1. Pyrimidine bases:
The pyrimidines are single ring compounds with nitrogen in positions 1′ and 3′ of a benzene ring. There are three pyrimidine bases in the nucleic acids. These are cytosine (C), Thymine (T) and Uracil (U).
Cytosine is found in both DNA and RNA while thymine is characteristic of DNA. Uracil is characteristic of RNA.
2. Purine bases:
The purines are double ring compounds. A purine molecule has a 5 membered imidazol ring joined to a pyrimidine ring at positions 4′ and 5′.
In nucleic acid, there are two purine bases, namely. Adenine (A) and Guanine (G). These are common to both DNA and RNA. The structural formulae of these bases are shown in Figure 10.1 (a).
In certain RNA molecules particularly the transfer RNA (found mainly in cytoplasm and nucleolus), a large proportions of bases are methylated, i.e., they are in the form of methyl adenine, methyl guanine and methyl cytosine.
Chemical Compounds in Chromatin: Class # 4. Pentoses:
Pentoses are 5 carbon ketosugars. They are of two types; one for each type of nucleic acid; ribose sugar in RNA and deoxyribose in DNA. In deoxyribose sugar, oxygen in the second carbon is lacking. Both ribose and deoxyribose have a pentagonal rings with 5 carbons.
Two carbons at 3 and 5 positions are linked to phosphoric acid and carbon 1 linked to the base. Deoxyribose is responsible for positive fuelgen reaction which is specific for DNA. The structural formulae of the two pentose sugars are given in Fig. 10.1 (b).
Chemical Compounds in Chromatin: Class # 5. Phosphoric Acid:
It links nucleotides by joining the pentose sugars of two consecutive nucleotides by phosphodiester bonds. These bonds are established between phosphoric acid and OH groups of pentose sugars of two consecutive nucleotides.
On one side of phosphate group, OH group of 3 ‘C of pentose in one nucleoside is involved in the formation of one ester bond and on the other side, OH group of 5’C of pentose sugar of next nucleoside forms a second ester bond [Fig. 10.1 (c)].
In this way, two of the three acid groups of a phosphoric acid are used in joining the sugars of two nucleosides. The remaining third acid group enables the molecule to form ionic bonds with basic proteins (i.e., histones and protamines).
The DNA and RNA differ from each other in the kinds of bases and sugars found in them.
Bases and sugars of RNA and DNA are listed in the following Table 10.1:
The different types of deoxyribonucleotides and ribonucleotides (ribotides) besides occurring in DNA and RNA respectively also occur in nucleoplasm and cytoplasm, but in their triphosphate forms.
Polynucleotide:
As stated earlier, the nucleic acids are polymeric molecules consisting of a number of nucleotides in series. The connecting links between consecutive nucleotides are of ester type in which the hydroxyl groups of 3 and 5 carbons of two different sugars form a double ester bond with phosphoric acid.
This is known as phosphodiester bond [Fig. 10.1 (c)]. In both DNA and RNA, the bases are attached to pentose moiety by a covalent bond between 1 ‘C of sugar and the 9-position nitrogen of the purines or 3-position nitrogen of pyrimidines.