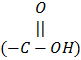An understanding of the mechanisms of toxicity is of practical and theoretical importance. Such information provides rational basis for interpreting descriptive toxicity data, estimating the probability that a xenobiotic will cause harmful effects, designing drugs and industrial chemicals that are less hazardous, and developing pesticides that are more selectively toxic for their respective target organisms.
As a result of the huge number of potential toxicants and the multitude of biological structures and processes that can be impaired, there are a tremendous number of possible toxic effects. Correspondingly, there are various pathways that may lead to toxicity (Fig. 18.1).
The most direct pathway occurs when the chemical causes toxicity by its mere presence at critical sites in the body without interacting with a target molecule (path A). This pathway is followed, for example, when agents precipitate in renal tubules and block urine formation. With this type of toxicity, delivery (step 1) is the most important consideration.
An example of a more complex route (path B) to toxicity is that taken by the fugu fish poison tetrodotoxin. After ingestion, this poison reaches the Na+ channels of motoneurons (step 1). Interaction of tetrodotoxin with this target (step 2) results in blockade of Na+ channels, inhibition of the activity of motor neurons (step 3), and ultimately skeletal muscle paralysis. No repair mechanisms can prevent the onset of such toxicity.
The most complex path to toxicity (path C) involves even more steps. First, the toxicant is delivered to its target or targets (step 1), after which the ultimate toxicant interacts with endogenous target molecules (step 2), triggering perturbations in cell function and/or structure (step 3), which initiate repair mechanisms at the molecular, cellular, and/or tissue levels (step 4).
When the perturbations induced by the toxicant exceed the repair capacity or when repair becomes malfunctional, toxicity occurs. Tissue necrosis, cancer, and fibrosis are examples of chemically induced toxicities whose development follow this four-step course (Fig. 18.1).
Present section deals with the mechanism (mode) of toxic action of the following commonly used toxicants:
1. Mechanism of action of some metals
2. Pesticides:
i. Mechanism of action of organochlorine insecticides.
ii. Mechanism of action of organophosphate insecticides.
iii. Mechanism of action of carbamates.
iv. Mechanism of action of pyrethroids.
3. Mechanism of action of carcinogens.
4. Mechanism of action of teratogen.
5. Mechanism of action of ionizing radiations.
1. Mechanism (Mode) of Toxic Action of Metals:
Various metals exert toxicity by the following means:
(A) By Affecting Permeability of Plasma Membrane and of Subcellular Organelles:
In general, some metals viz., Cu, Hg etc. interact with phosphate fractions of plasma membrane, bind firmly with thio ligands and exert conformational changes in the membrane. Such changes may either activate a membrane bound enzyme or may activate an ion channel exerting a series of cell responses resulting in cell damage.
Lipo-protein molecules are the chief component of plasma membrane. Various metals like Cu, Ar, Mn, Ni, Co and Hg may bind with phospholipid fraction of plasma membrane. Hg and Pb, by their binding, increase passive alkaline ion permeability. Hg+2 block glucose transfer into R.B.C.
(B) By Stabilizing Tissue Constituents and Cellular Organelles:
A number of metals bind with tissue components, stabilize them and thus exert toxicity. For example, Cu and Hg bind with collagen protein; Co, Ni, Cu, and Zn form covalent linking with protein by binding with imidazole nitrogen of histidine and SH groups of cysteine. Hg and Ag bind with Hb irreversibly and form Hg-Hb, and Ag-Hb complexes, respectively, and then obstruct O2– carrying function of hemoglobin.
Hg also binds serum albumen and stabilizes it.
Metals show affinity with NH2 group. These bind with NH2 groups of protein causing irreversible change.
The affinity of metals for NH2 group is in the following order:
Hg > Cu > Ni > Pb > Zn > Co > Cd > Mn
(C) By Inactivating Enzymes and Cell Constituents by Binding with Functional Groups:
Metal ions bind with functional groups or ligands present in biologically active molecules and form coordinate covalent complexes with nitrogen ligands or other groups such as – SH, -OH, -NH2, -COOH, -PO4-3 etc. and exert toxicity.
i. Lead binds with -SH2, -NH2 inside hemoglobin and causes lysis of porphyrin. It also binds with PO4-3 ligands and produces deleterious effects.
ii. Cd binds with -SH group of glutamic dehydrogenase and consequently causes irreversible inactivation of the enzyme and inhibits alkaline phosphatase and ATPase activity.
iii. Arsenic binds with -SH group of hemoglobin and causes hemolysis.
In general, toxic metals and metallic compounds attack the active sites of enzymes, inhibiting essential enzyme function. Heavy metal ions in particular, e.g., Hg2+, Pb2+ and Cd2+ act as effective enzyme inhibitors. They have affinity for sulphur containing ligands e.g., -SCH3 and -SH in methionine and cystein amino acids, which are part of the enzyme structure.
Metalloenzymes contain metals in their structures. Their action is inhibited when one metal ion of a metalloenzyme is replaced by another metal ion of similar size and charge. Thus Zn++ in some metalloenzymes is substituted by Cd++ which leads to Cadmium toxicity.
The enzymes inhibited by Cd++ include adenosine triphosphatase, alcohol dehydrogenase, amylase, carbonic anhydrase and glutamic – oxaloacetic transaminase. Pb+2 inhibits acetylcholinesterase, carbonic anhydrase, cytochrome oxidase, alkaline phosphatase, ATPase and some of the key enzymes in the synthesis of heme.
D. By Causing Irreversible Damage to Nucleic Acids after Binding with them:
Many metals, for example, Cd, Pb and Al, stabilize RNA. Certain metals like Zn cleaves 5 phosphate bond in RNA, resulting in depolarization. Some metals, for example, Cu unwinds DNA and disrupts it.
Few metals like Zn+2 and Co+2 compete with Mg+2 ions necessary for the incorporation of deoxyribonucleotide and ribonucleotides in DNA and RNA synthesis, respectively, causing damage to DNA replication and transcription. Cu and Cd compete with Mg+2 of RNA polymerase enzyme system causing damage to RNA synthesis.
2. Mechanism of Action of Pesticides:
I. Mechanism of Toxic Action of Organochlorines:
The chlorinated hydrocarbons are stimulants of the nervous system. Their mode of action is similar in insects and humans. They affect nerve fibres along the entire length by disturbing the transmission of the nerve impulse. More specifically, the members of this group of insecticides disrupt the Na/K balance that surrounds the nerve fibers. The result of this imbalance is a nerve that sends transmissions continuously rather than in response to stimuli.
Despite the similarity of many of the compounds within each of the three subgroups, the individual toxicities vary greatly. The compounds also vary greatly in their ability to be stored in tissue. For example, the structure of methoxychlor is very similar to DDT, but its toxicity is far lower, as its tendency to accumulate in fatty tissue.
Storage in fatty tissue is actually a strategy that the body uses to remove toxic materials from active circulation. Fatty storage prevents the toxic agent from reaching in the circulation and consequently on various organs like liver, kidney etc.
DDT type insecticides interact with the neuronal membrane by altering the membrane permeability (transport) for potassium and sodium and the calcium mediated processes. By inhibiting these functions, the repolarization of the nerved is distributed resulting in hyper-excitability.
Cyclodines and cyclohexane compounds have a central nervous system stimulating mode of action. These compounds antagonize the neurotransmitter, γ – aminobutyric acid (GABA), permitting only partial repolarization of the neuron and thus uncoordinated nervous excitation.
Owing to their lipophilicity, organochlorine compounds are partitioned and stored largely in adipose tissue, where they are biologically inactive. There is an equilibrium between body fat and free circulating compounds. Redistribution and mobilization of fat, for example, due to disease, ageing or fasting may result in mobilization of stored organochlorines in quantities that may lead to manifest toxicity. Owing to the different patterns of use of organochlorine insecticides in industrialized and developing countries, the pattern of distribution of the residues in human fat (including breast milk) differs between countries.
Summary of Mechanism of Action of OCIs:
CHIs act as diffused CNS stimulants. They do not act upon any specific receptors.
Their possible mechanism of action may be:
a. They change the electrical activity in the CNS by altering Na+ and K+ flux across the nerve cell membranes.
b. They increase the concentration of free ammonia and glutamine in the brain.
c. They inhibit Na+, K+ and Mg++ ATPase in the nerve endings of CNS.
d. Cyclodienes inhibit GABA receptor mediated CI– transport and clamodulin regulated Ca++ pump activity in the brain.
II. Mechanism of Toxic Action of OPIs (Organophosphate Insecticides):
The OPIs in general are less persistent than the OCIs, a property due to which they are preferred to the OCIs. In principle, OP compounds react with the active site of AChE (a serine hydroxyl group). In other words, all OP compounds are anticholinesterase agents, i.e., they inhibit the enzyme cholinesterase (ChE) at the neuromuscular junction.
During normal muscle contraction, the cholinergic nerve fibres of autonomic system liberate acetylcholine (ACh) at the neuromuscular junction. The ACh excites muscle fibres and, consequently, muscle contraction takes place. Very soon ACh is splitted by ChE present at the neuromuscular junction into choline and acetyl CoA.
Actually splitting of ACh by ChE prevents its accumulation at the neuromuscular junction and thus assists in muscle relaxation after each contraction:
In its usual course of action, the ChE first gets acylated by losing one H+ and then fastly recovers by using one molecule of metabolic HOH.
Actually the OP compounds are structurally similar to ACh. At the enzyme site, they compete with ACh and bind with ChE, thus inhibiting its normal function. The OP compounds phosphorylate the ChE and form a stable complex. Ultimately the enzyme fails to recover quickly.
These events bring about an accumulation and consequently increase of ACh at the neuromuscular junction, which in turn, respectively, excites the muscle fibers and produces repetitive muscle contraction that causes tremors in the body muscles of insect and the insect finally dies of exhaustion.
III. Mechanism of Toxic Action of Carbamates:
The primary way that insecticidal carbamates work on both target and non-target species is through the inhibition of the enzyme acetylcholinesterase. Acetylcholine (ACh) is a substance that transmits nerve impulse from a nerve cell to a specific receptor such as another nerve cell or a muscle cell. Acetylcholine, in essence, acts as a chemical switch. When it is present (produced by the nerve cell) it turns the nerve impulse on. When it is absent, the nerve impulse is discontinued.
The transmission ends when the enzyme acetylcholinesterase breaks down the ACh into choline and acetic acid. Without the action of this enzyme, ACh builds up at the junction of the nerve cell and the receptor site, and the nerve impulse continues. Carbamate insecticides block (or inhibit) the ability of this enzyme, (acetylcholinesterase) to break down the ACh and end the nerve impulse.
Carbamate inhibition of acetylcholinestrase is reversible process. Estimate of the recovery time in humans range from immediate up to 4 days, depending on the dose, the specific pesticide, and the method of exposure. The breakdown of carbamate compounds within an organism is a complex process and is dependent on the specific pesticide structure.
In nutshell the mode of action of carbamates, like OPIs is the inhibition of acetylcholinesterase. Carbamates are potent reversible inhibitors of cholinesterase. In addition, all the carbamates inhibit aliesterase of insects, but they kill insects and mammals entirely by cholinesterase inhibition.
The mechanism of inhibition of AChE and its reactivation is shown in Fig. 18.2 taking an example of carbaryl, a widely used carbamate (Fig. 18.2):
IV. Mechanism of Toxic Action of Pyrethroids:
The Type I esters affect the sodium channels in nerve membranes with prolongation of sodium influx causing repetitive neuronal discharge and prolonged after potential but no severe membrane depolarization. The type II α cyanoesters lead to greater and more prolonged sodium influx with persistent membrane depolarization and eventually nerve blockade.
Whereas the first group exerts its main effects on synaptic transmission causing hyperexcitabilty and tremor, the second group shows its first effects on the sensory nervous system. Other actions include inhibition of Na+/Mg+2 ATPase (adenosine triphosphatase) and alteration of calcium and chloride ion homeostasis.
3. Mechanism of Action of Environmental Carcinogens:
Chemical carcinogenesis is a multistage process. Carcinogenic chemicals act either by covalently binding with genetic macromolecules or by promoting the process. The former type of chemicals are known as genotoxic carcinogens because they, or their reactive metabolites, react with the genetic materials.
After the interaction between a genotoxic carcinogen and the DNA, the affected cell either dies or reverts to a normal cell through error-free DNA repair. If neither of these events occurs, the carcinogenic initiation becomes irreversible after the cell undergoes replication. Such an initiated cell may remain dormant for a long period of time before it becomes a tumour through cellular proliferation.
Initiated cells remain dormant probably because of the suppressant influence of the surrounding normal cells. This influence is apparently reduced under varying conditions. These include cell removal (e.g., partial hepatectomy), cell killing (resulting from cytotoxic chemicals, viruses, radiation), growth factors (e.g., hormones), and exogenous factors.
Epigenetic carcinogens increase the tumour yield by promoting the replication of cells initiated by genotoxic carcinogens, or by augmenting the available amount of genotoxic carcinogens and/or their metabolites at the site of action. In long-term carcinogenesis studies, the animals given epigenetic carcinogens develop more tumours than the controls, or the tumours appear earlier, presumably because of promotion of neoplastic cells arising from inherited genetic defects or from exposure to unknown environmental genotoxic carcinogens.
4. Mechanism of Action of Teratogens:
A variety of chemicals have been known to be teratogenic in animal models.
In view of the great diversity of the properties of these agents, it is not surprising that many different mechanisms are involved in their teratogenic effects as given below:
i. Interference with Nucleic Acids:
Various teratogenic agents interfere with nucleic acids functioning viz., replication, transcription, or translation. These include alkylating agents, antimetabolites, intercalating agents and amino acid antagonists. Some of these chemicals are active; others require bio-activation, such as aflatoxin and thalidomide. Although some chemicals, e.g., carbon tetrachloride and nitrosamines, also yield reactive metabolites, however, their reactive metabolites are too unstable to reach the embryo. Therefore, these toxicants are not potent teratogens.
ii. Deficiency of Energy Supply and Osmolarity:
Certain teratogens may affect the energy supply for the metabolism of the organism by restricting the availability of substrates either directly (e.g., dietary deficiencies) or through the presence of analogs or antagonists of vitamins, essential amino acids, and others. In addition, hypoxia and agents inducing hypoxia (CO, CO2) can be teratogenic by depriving the metabolic process of the required oxygen and probably also by the production of osmolar imbalances. These can induce edema and hematomas, which, in turn, may cause mechanical distortion and tissue ischemia.
Inhibitors of enzymes, e.g., 5-fluorouracil, can induce malformation through interference with differentiation or growth by inhibiting thymidylate synthetase. Other examples include 6-aminonico- tinamide, which inhibits glucose-6 phosphate dehydrogenase, and folate antagonists, which inhibit dihydrofolate reductase.
Hypervitaminosis A may be associated with ultrastructural damage to cellular membranes in rodent embryos, a mechanism that can explain the teratogenicity of vitamin A. Physical agents that may cause malformation include redaction hypothermia and hyperthermia and mechanical trauma.
It shall not be out of place to mention that the mode of action of many teratogens is as yet uncertain. Furthermore, a potential teratogen may or may not exert teratogenic effects depending on such factors as bio-activating mechanism, stability of the reactive metabolites, ability to cross the placental barrier, and detoxifying capability of the embryonic tissues. Appropriate experimental testing for the teratogenicity of toxicants is, therefore, essential.
5. Mechanism of Action of Ionizing and Non-Ionizing Radiations:
When ionizing radiations penetrate the living tissue, it wreaks havoc on the atoms and molecules in its path. Actually, when a water molecule in the cell is irradiated, an electron is knocked out of its orbit. The ejected electron may then attach to a normal water molecule creating instability.
Such unstable water molecules (HOH) split into hydrogen ions (H+), hydroxyl ions (OH– ) and the free radicals -H* and OH*. Radiation also produces a host of several species e.g. H2, H2O– , H2O+, H2+, HO2, H3O–, e–, e+ and H2O2. The free radicals are extremely reactive. They react with protein molecules in the cell, setting a chain of events that can destroy living cells or make them to function abnormally.
The ionic species deactivate the enzymes by dissociating the S-H-S hydrogen bond. Consequently, with enzyme inhibition, cell growth may continue but cell division and multiplication may be stopped. Proteins are mainly the body builders that play an important role in the formation of plasma membranes.
Radiation exposure may damage the plasma membrane by making it permeable, while the large doses of ionizing radiations can kill quickly of inflict severe damage. Even the lower doses can initiate cancer throughout the body. Radiation also results in abnormal interchange of materials through an imperfect plasma membrane causing temporary or permanent injury in the body.
Studies of survivors who have received significant doses such as atom bomb survivors, uranium miners and radium watch dial painters etc. showed that damage depends on how the victims were exposed. Experiments revealed that human organs can repair some radiation damage. The sensitivity to radiation damage appears to be directly proportional to the cell’s reproductive capacity and inversely proportional to its degree of differentiation.
Mechanism of Reaction of Ionizing Radiation:
Tiny radiation particles viz., a-particles damage the cell as shown in Fig. 18.3:
(i) When a beam of high energy positively charged alpha particles penetrates a living cell, it disrupts the atoms and molecules in its path. Water molecule (say A), for example, can be dissociated by the alpha particle’s positive charge.
(ii) The alpha radiation’s strong positive charge strips the water molecule of an electron (e–) forming ions (B). The water molecule (H2O) being neutral in character acquires a positive charge (H2O+) and destabilise its relationship with neighbouring molecules.
(iii) A deoxyribonucleic acid (DNA) molecule (C) can also be broken apart or altered by alpha particles or ions. Sometimes the DNA’s genetic code is scrambled which then reproduces in later generations.
(iv) In this step damaged strands of DNA can cause chromosomes to break apart and then recombine in an abnormal manner (D). Unless the body’s repair system can isolate and treat or overcome the damage, the cell may eventually die (E), often within few hours.
(v) Radiation may also bring chronic alterations in DNA molecules which, if not destroyed completely, may reproduce abnormally for several years, spawning cancerous cells and eventually resulting in acute tumours (F).
How do Various Particle Radiations Differ in their Hazard?
Radioactive elements emit three types of radiations:
(i) Alpha particles (positively charged).
(ii) Beta particles (negatively charged), and
(iii) Gamma rays without any charge and identical to X-rays.
Actually alpha particles dissipate their energy quickly and hence have less penetrating power and pose minimum hazard. However, when alpha emitters are ingested, they can be very bad since for equal energy they can produce more ‘ion-pairs’ than either beta or gamma rays.
Beta (β) particles are generally more penetrating than alpha or X-rays. β -emitters external to the body are more dangerous than alpha emitters; since β – rays can penetrate from a few millimeters to a centimeter or so under the skin. When β -emitters are ingested they are more hazardous than they are externally but are comparatively less than the ingestion of alpha emitters because of the lesser specific ionization by beta particles.
Gamma rays have the highest penetrating power and so, like X-rays, are most dangerous. Gamma- rays may destroy tissues and inflict serious burns quite rapidly.
Both gamma and X-rays interact with living matter in three ways:
(i) By ion-pair production (i.e., high hγ on high atomic weight atoms to produce e– and e+).
(ii) Photoelectric way (i.e., ejection of e– by low hγ on high atomic weight atoms)
(iii) Compton (i.e., moderate hγ on atom of any atomic weight to produce e– and longer λ).
The charged particles so formed then go on to excite and ionize the biological medium. Since gamma-rays and X-rays can penetrate deeply in the body tissue, they constitute a hazard for the entire body. Incidentally, gamma-emission frequently accompanies alpha or beta emissions.
II. Mechanism of Non-Ionizing Radiations:
Modern life and radiations seem to increase the risk of radiative pollution. Of all the nonionizing radiations including infrared, radio waves, microwaves, radar etc., the action of ultraviolet (UV) radiations has been extensively studied.
In body cells, the protein and nucleic acid are chiefly responsible for the absorption of radiation. In the region of 240 nm to 280 nm wavelength, the absorption by nucleic acid is 10 to 20 times greater than that by proteins of the same weight.
1. Normally the pyrimidines (bases found in DNA) Thymine (T) and cytosine (C), undergo photochemical reactions much readily than the purines, adenine (A) and guanine (G), on absorption of UV radiations. During the reaction, all the DNA molecules get charged and acquire the excited state. At this stage, molecules are capable of undergoing further reactions leading to mutations.
2. UV radiations are thought to trigger two distinct immunological effects. One is confined to patches of skin that are actually irradiated while the other damage is caused to the immune system as a whole.
3. UV radiations cause the blood vessels near the skin’s epidermis to carry more blood causing the skin hot, swollen or sun burns.
4. Serious skin cancers including the basal cell carcinoma, squamous cell carcinoma and melanoma are rapidly climbing the list of human diseases caused by UV radiations.
5. It has been observed that closer a fair-skinned person lives to the equator, the more likely he is to get non-melanoma cancer by UV rays.
6. UV rays can also be absorbed by lens and cornea in the eye leading to photo-keratitis and cataracts. Since the radiation is not sensed by the visual receptors of eye, the damage is done without the individual knowing about its hazards.
These radiations are also associated with DNA breakage, inhibition and alterations of its replications and formation of DNA adduct which has been implicated in premature ageing and finally death of the cells.