Do you want to create an amazing science fair project on water pollution ? You are in the right place. Read the below given article to get a complete idea on water pollution: 1. Meaning of Water Pollution 2. Sources of Water Pollution 3. Types 4. Effects 5. Prevention and Controls.
Contents:
- Science Fair Project on the Meaning of Water Pollution
- Science Fair Project on the Sources of Water Pollution
- Science Fair Project on the Types of Water Pollution
- Science Fair Project on the Effects of Water Pollution
- Science Fair Project on the Prevention and Controls of Water Pollution
Contents
Science Fair Project # 1. Meaning of Water Pollution:
To understand water pollution, it is essential to know the chemical phenomena that occur in water. The chemical phenomena includes acid-base reaction, solubility, oxidation-reduction and complexion reactions. Acid-base phenomena in water involve loss and acceptance of H+ ions.
Many species act as acids in water by releasing H+ ions, others act as bases by accepting H+ ions, and the water molecule itself does both.
An important species in the acid-base chemistry of water is bicarbonate ion, HCO3, which may act as either an acid or a base:
Various gases viz., O3, CO2, NH3, CI, SOX, NOX etc. are soluble in water along with different salts and mostly maintain an equilibrium of ions.
Oxidation-reduction phenomena are highly significant in the environmental chemistry of natural water and wastewater. In a lake, for example, the reduction of oxygen (O2) by organic matter (represented by CH20). [CH2O + O2 → CO2 + H2O], results in oxygen depletion that can kill fish.
The rate at which sewage is oxidised is crucial to the operation of a waste treatment plant. Reduction of insoluble iron (III) to soluble iron (II), [Fe (OH)3(S) + 3H+ + e → Fe2+ + 3H2O] in a reservoir contaminates the water with dissolved iron, which is hard to remove in the water treatment plant. Oxidation of NH4 to NOJ in water converts ammonium nitrogen to nitrate, a form more assailable by algae in the water.
NH4 + 2O2→ NO3 + 2H+ + H2O
The chelating agents are common potential water pollutant and thus forms various metal complexes.
Science Fair Project # 2. Sources of Water Pollution:
The common sources of water pollution are:
i. Sewage and Other Wastes:
Sewage is a water-borne waste consisting of domestic effluent (home and animal) and of food processing plants. Contamination of fresh waters and offshore marine waters by sewage is of common occurrence as the drains consisting these wastes drain into these impoundments.
It consists of human excreta, paper, cloth, soap, detergent, food items etc. Domestic sewage and other waste waters are made up of 99.9 per-cent water and 0.02-0.04 per-cent solids which are mostly biodegradable pollutants.
Due to uncontrolled dumping of wastes into ponds, lakes, rivers etc., recycling of these ingredients is not possible as the input exceeds the decomposition or dispersal capacity of these water bodies. The decomposition rate by aerobic microbes decreases and the self-purifying capacity of the water is lost. Water becomes unfit for drinking and for other domestic uses.
ii. Industrial Wastes:
All industrial plants produce some organic and inorganic chemical wastes. They are present in effluents from breweries, tanneries, dyeing textiles, paper and pulp mills, steel industries, mining operations, and coal washeries, various workshops (rail etc.), synthetic material plants for drugs, fibres, rubber, plastics etc.
The industrial wastes of these include pollutants such as oil, grease, plastic, metallic waste (Na, Cr, Cd, Hg, Pb, and Cu etc.), suspended solid, phenol, toxin, acid, salt, dye, cyanide etc.
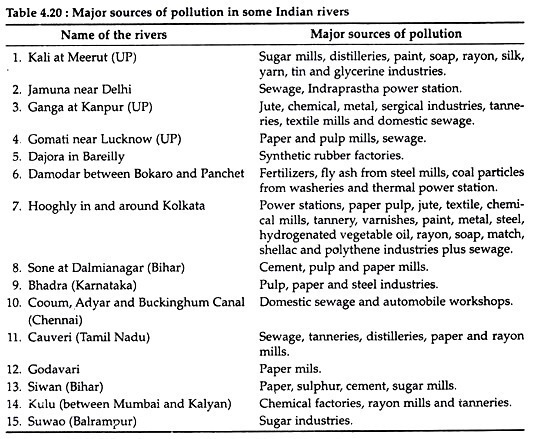
Most of these wastes are dumped indiscriminately into rivers (Table 4.20), streams, ponds, lakes etc. For example, 80 million litres of toxic effluents are discharged into the Periyar River each day by the industrial units of greater Cochin area. Many of these wastes are not readily degradable.
These chemicals changes the pH of water and increase COD and anoxic conditions of the water bodies. It has been observed that the pollution load contributed by a small scale industry may be equal to that contributed by the sewage from a large city.
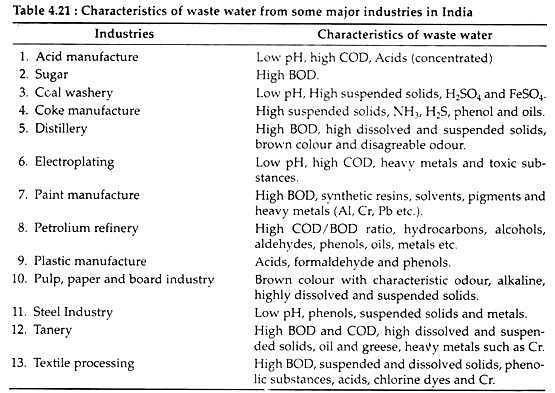
The nature and quality of wastes (Table 4.21) differ from industry to industry depending upon the nature of raw materials, process used and products manufactured and recovered. The industrial wastes must be removed and transported to some place for subsequent disposal or treatment. However, accidental spills, leakage etc. may occur in spite of best efforts which may lead to grave consequences.
iii. Thermal Wastes:
Coal or oil or nuclear fired steam power plants are associated with thermal pollution, as heat here acts as a pollutant. In thermal power plant the quantity of waste water is the highest in the country. Most electricity generating plants operate through the thermodynamic process known as Rankine power conversion cycle. A plant generally operates at 40% efficiency.
It generates 16.7 joules of waste heat for every 41.8 joules of fuel burnt. The condenser coils of the plant are cooled with water from nearby river, lake or pond and the heated water is discharged back into the same impoundment. In the process it raises the temperature by 10°C.
The hazards of this rise in temperature are:
a. Acceleration of chemical reactions.
b. The hot water kills some plants and animals.
c. Early hatching of fish eggs.
d. Failure of trout eggs to hatch.
e. Salmons fail to spawn.
f. Increase in BOD.
g. Change in seasonal and diurnal behaviour and metabolic responses of organisms.
h. Shift in aquatic organisms towards more, heat tolerant forms (decrease in species diversity).
i. Migration of some aquatic forms to less heated water.
j. The most damaging environmental effect is that large number of organisms may be sucked in through the water intake, thereby destroying the floral and faunal structure of the water body.
However, this heated water can be put into use through proper planning. Power stations could be linked to sewage treatment plants. Mixing hot power station effluents with raw sewage accelerates the treatment process.
While the circulation of partially treated sewage effluent around the cooling tower accelerates the conversion of organic nitrogenous compounds and ammonia to nitrate and nitrite. Heated water also can be used to heat glasshouses for production of high value crops such as tomatoes, bananas etc.
iv. Radioactive Materials:
Human activities are responsible for radioactive pollution, which are:
a. Mining and processing of ores to produce useable radioactive substances.
b. Radioactive materials used in nuclear weapons.
c. Radioactive materials used in nuclear power plants.
d. Use of radioactive isotope in medical, industrial and research applications.
Nuclear weapons testing, leakage etc. give rise to radioactive fallouts. It has far-reaching effects on the environment and mankind. For example, Sr90, a radioactive fallout, is chemically similar to Ca and accompanies Ca in soil. It then enters into plants, animals and finally into the bones and teeth of man. The presence of Sr90 leads to disorders in blood cell formation and causes anemia.
v. Detergents:
Sodium alkyl sulphate is a synthetic detergent included in dish detergent, shampoo and commercial toothpaste and is responsible for causing foam. Residues of Alkyl benzene sulphate (ABS) are non-biodegradable.
Another effective and most widely used compound today is sodium tripolyphosphate (STP). Huge amount of these synthetic detergents are added indiscriminately into water bodies, due to their increased use in domestic and industrial processes.
Synthetic detergents cause water pollution in concentration as low as 1 ppm. Most of the currently used detergents are biodegradable and are reduced to the basic inorganic chemical components. It contains much phosphorus which stimulates algal growth and thus hastens eutrophication.
These algal blooms may even persist for weeks. They impart odour, colour and unpleasant taste to the water and thus cause inconvenience in water supply, fish farming and make the use of water ineffective.
Science Fair Project # 3. Types of Water Pollution:
The followings are some of the types of water pollution:
i. Ground Water Pollution:
The ground water is most prime water which have multipurpose uses ranging from drinking to industrial and agricultural uses. The quality requirement varies distinctly with respect to the specific uses. For instance drinking water must have specified quality (Table 11.1), which is not at all essential for industrial purposes or other domestic uses.
Though ground water appears to be less prone to pollutant mixing yet there are a number of potential sources of ground water pollution (Table 11.2). Ground water contamination with arsenic, fluoride, and nitrate recently possess serious health hazards to large sector of communities all over the world. Most illustrated accounts of ground water pollution is further described in 11.2.
ii. Surface Water Pollution:
Major lakes, rivers, reservoirs of the world are now getting polluted by various ways (Table 11.3), and thereby posing threat to the survivability of the life systems on these diverse water bodies. There are a number of routes of entry of pollutants to the surface water.
Regular monitoring of these contaminating routes and their effective protective action plan has to be evolved for better conservation of surface water resources in future. Water quality status of some rivers of India is depicted in Table 11.4.
Science Fair Project # 4. Effects of Water Pollution:
The effects of water pollution are herewith:
i. Sewage Pollution:
Contamination of fresh-waters and shallow offshore seas by sewage is a common occurrence. Sewage generally includes biodegradable pollutants such as human faecal matter animal wastes and certain dissolved organic compounds (e.g., carbohydrates, proteins, urea, etc.) and inorganic salts such as nitrates and phosphates of detergents and sodium, potassium, calcium and chloride ions.
Under natural processes most of the biodegradable pollutants of sewage are rapidly decomposed, but when they accumulate in large quantities, they create problem, i.e., when their input in the environment exceeds the decomposition capacity of the latter.
Most cities of developed countries like USA, Britain, etc., and some cities of developing countries like India have evolved certain engineering systems, such as, septic tanks, oxidation ponds, filter beds, waste-water treatment plants and municipal sewage treatment plants for the removal of many harmful bacteria and other microbes, organic wastes and other pollutants from the sewage, before it is tipped into river or sea.
The sewage treatment is usually performed in following three stages:
(a) Primary treatment, which removes large objects and suspended un-dissolved solids of raw sewage and converts them into a biologically inactive and aesthetically inoffensive state, called the sludge, a valuable fertilizer.
(b) Secondary treatment, which supplies aeration and bacteriological action to decompose organic compounds into harmless substances such as carbon dioxide, sulphate and water During later stages of secondary treatment, whole waste water is chlorinated (i.e., treated with chlorine) to reduce its contents of bacteria.
(c) Tertiary treatment, which removes nitrates and phosphates and releases pure water. These three stages of sewage treatment have become increasingly expensive and only in most advanced countries all the three treatments of sewage are done.
However, most Indian cities either lack any sewage or waste-water treatment plant or have inadequate sewage treatment facilities. Consequently, normally and especially during heavy downpour and floods raw sewage or incompletely treated sewage is dumped into rivers which causes severe water pollution problems.
ii. Industrial Pollution:
Most of the Indian rivers and fresh-water streams are seriously polluted by industrial wastes or effluents (Table 15.2 and 15.3) which come along waste waters of different industries such as petro-chemical complexes, fertilizer factories, oil refineries, pulp, paper, textile, sugar, steel mills, tanneries, distilleries, drugs, fibres, rubber, plastic, etc.
All these chemicals of industrial wastes are toxic to animals and may cause death or damage to different body systems of the aquatic fauna and flora.
iii. Thermal Pollution:
Various industrial processes may utilize water for cooling and resultant warmed water has often been discharged into streams or lakes. Coal or oil-fired generators and atomic energy plants generate large amounts of waste heat which is carried away as hot water and cause thermal pollution.
The thermal pollution produces distinct changes in aquatic biota. However, the thermal pollution can exert a disruptive effect on aquatic ecosystem.
iv. Destruction of Aquatic Ecosystem:
Organic and inorganic wastes decrease the dissolved oxygen (DO) content of water bodies.
Water having DO content less than 8.0 mg L-1 is considered contaminated. Heavily polluted waters have DO content less than 4.0 mg L-1.
DO content water is important for the survival of aquatic organisms.
The turbidity, photosynthetic activity, oxygen consumption by organisms and decomposition of organic matter are the factors that determine the amount of DO present in water.
Higher amounts of organic waste increase the rate of oxygen consumption, thus causes drop in DO content of water.
The demand of oxygen is directly related to increasing input of organic wastes and is referred to as biochemical oxygen demand (BOD) of water.
BOD is a measure of oxygen required by aerobic decomposers for the biochemical degradation of organic materials (biodegradable materials) in water.
Higher the BOD, lower would be dissolved oxygen (DO).
Chemical oxygen demand (COD) is also a measure of pollution load in water. This is measure of oxygen equivalent of the requirement for oxidation of total organic matter, (i.e., biodegradable and non-biodegradable) present in water.
Thus, contamination of water bodies by pollutants reduces DO content, and therefore, sensitive organisms, such as plankton, mollusc and fish are eliminated.
In such state, only a few tolerant species, like annelid Tubifex and some insect larvae, survive in highly polluted, low DO water, and they are referred to as indicator species for polluted waters. Biocide residues, PCBs and heavy metals like Hg, Cd, Pb, Cu, As, etc., can eliminate different species of organisms.
Higher the temperature of water, lower is the rate of dissolution of oxygen in water.
However, hot wastewaters discharged from industries, when added to water bodies, cause lowering of DO content of water.
v. Biological Magnification:
The phenomenon through which certain pollutants are accumulated in tissues in increasing concentration along the food chain, is referred to as biological magnification. Such pollutants are non-biodegradable, such as DDT. Once they are absorbed by an organism, they cannot be metabolised and broken down or excreted out.
Such pollutants (non-biodegradable), generally get accumulated in fat containing tissues of the organism.
For example, DDT an insecticide, is sprayed on water bodies to check the growth of mosquitoes.
In an island of USA, after regular DDT spraying for few years, the population of fish-eating birds decreased. Later, this was found that the concentration of DDT had increased about 800 times in the phytoplankton relative to the concentration in water.
However, zooplankton contained about five times greater DDT than phytoplankton.
In different fish, the DDT concentration increased 9 to 40 times, relative to the concentrations in zooplankton.
Birds showed about 25 times greater DDT concentration relative to that in fish.
Many other persistent pesticides and radionuclides also show biological magnification.
vi. Eutrophication:
Here, instead of inorganic nutrient input with the inflow of waste water, decomposition of organic wastes, increases the nutrient content of the water bodies.
The excess of nutrients causes profuse growth of algae, especially the blue-green algae (cyanobacteria). Such profuse growth of algae is called algal bloom.
Such algal blooms completely cover the water surface.
The blue-green algae release toxins in water, and sometimes cause deficiency of oxygen in water. Therefore, in bloom-infected water body the growth of other fresh water is checked due to toxins, and aquatic animals may die because of toxic effect or lack of oxygen.
The process of enrichment of nutrients of water, and then loss of species diversity is known as eutrophication.
vii. Degradation of Human Health:
Domestic sewage contains pathogens, such as virus, bacteria, parasitic protozoans, and several types of worms.
Contaminated water with above mentioned pathogens causes several water borne diseases, like jaundice, cholera, typhoid, amoebiasis, etc. The contamination makes the water unfit for drinking purpose. Such water cannot be used even for bathing, swimming and irrigation purposes.
On the other hand, the heavy metal contamination of water causes serious ailments of human beings. Mercury poisoning, also known as Minamata disease is caused by consumption of fish captured from Hg-contaminated Minamata Bay in Japan. This disease was detected for first time in 1952.
Here, mercury compounds in wastewater are converted by bacterial action into extremely toxic methyl mercury. This may cause numbness of limbs, lips and tongue of humans. Deafness, blurring of vision and mental derangement are also caused by methyl mercury.
Cadmium, also a heavy metal causes itai-itai disease, also known as ouch-ouch disease, a painful disease of bones and joints. Cadmium (Cd) is also responsible for causing cancer of liver and lungs.
viii. Groundwater Pollution:
At many places in India, even the groundwater is contaminated due to seepage from industrial and municipal wastes and effluents. Sewage channels and agricultural run-off are also responsible for groundwater contamination.
For example, excess nitrate in drinking water is dangerous for human health, and may cause fatal diseases of infants. The nitrate reacts with haemoglobin of blood and forms non-functional methaemoglobin that weakens the oxygen transport. This is called methaemoglobinemia or blue- baby syndrome.
On the other hand, excess fluoride in drinking water causes teeth deformity. Excess of fluoride also causes a famous disease of bones, known as skeletal fluorosis in which bones become hardened and stiff, and joints painful.
In many parts of India, groundwater is contaminated with arsenic, mainly from naturally occurring arsenic in bedrocks, particularly in West Bengal. Excess of arsenic in water causes black-food disease. Arsenic also causes diarrhoea, peripheral neutritis, hyperkeratosis, and also skin and lung cancers.
Science Fair Project # 5. Prevention and Control of Water Pollution:
For the prevention of water pollution, most cities in India have evolved certain engineering systems such as septic tanks, oxidation ponds, filter beds, waste water treatment plants and municipal sewage treatment plants.
The various techniques used for control of water pollution are:
i. Stabilisation of the Ecosystem:
To control water pollution this is the most scientific way. This includes reduction in waste input, harvesting and removal of bio-mas, trapping of nutrients, fish management and aeration. The species diversity and ecological balance is restored through various biological as well as physical methods.
ii. Recycling and Re-Utilisation of Wastes:
Industrial effluent, municipal sewage, thermal pollution and other wastes can be recycled for beneficial use. Waste treatment plants have been installed at places where domestic as well as industrial effluents have been treated and the treated waste water has been used to advantage in aqua-culture and agriculture.
iii. Banning of Xenobiotics:
The various xenobiotics — synthetic herbicide and pesticides which have carcinogenic effect and are non-degradable — must be banned. Efficient and optimum use of organic manures should be encouraged.
iv. Removal of Oil:
Oil slicks can be got rid of by spraying detergents which break up the oil into drops. However, this is a crude method as detergents are more dangerous than oil and they kill much more marine organisms than oil.
A waste product from the paper industry resembling that of sawdust, called bregoil, has been successfully employed which can rapidly absorb oil. Recently, through genetic engineering, a strain of bacterium (Pseudomonas putida) has been created which has the ability to break down octane, xylene and camphor.
v. Biological Purification of Waste Water:
Water hyacinth (Eichhornia crassipes), an aquatic weed, has been used for purifying domestic and industrial waste water. This weed has tremendous capacity to accumulate heavy metals and even radioactive elements.
It is also efficient in absorbing nitrogen, phosphorus, and similar other chemical pollutants. It reduces COD, BOD and organic carbon. This plant has also been used as a new source of food, fertiliser and in production of biogas.
vi. Removal of Eyanides and Heavy Metals:
Waste water containing cyanides and heavy metals can be purified by utilizing certain bacteria which can not only withstand but can also grow as high concentration of cyanides. It can also absorb the cyanide. Bacteria are introduced in several rotating discs of the plants processing units.
These bacteria can pick up zinc, iron and other heavy metals from the wastes that pass over the plates. The water that comes out is then passed through clarifier, filtered and then released into the stream or river.
vii. Removal of Other Pollutants:
Other pollutants can be removed by processes devised by the Council of Scientific and Industrial Research, New Delhi.
(a) Ammonia can be removed by ion exchange technique. This is done by using a weak acidic cation exchange, which removes NH3 in the form of ammonium sulphate. This can be utilized as a fertilizer.
(b) Mercury can be removed by using mercury selective ion exchange resin.
(c) Phenolic from waste waters of pulp and paper mills, carbonization plants, petroleum refineries, tanneries and rain plants can be removed by the use of polymeric absorbents.
(d) Sodium salts can be removed by reverse osmosis method.
(e) Decolourisation of waste water from printing and sari dyeing industries can be done by an electrolyte decomposition technique.
vii. Utilisation of Solar Power:
Recent research developments have revealed the utilization of solar power for cleaning up polluted waters. A combination of sunlight and a catalyst, such as titanium dioxide, can break down chemical toxicants of water.
ix. Proper Implementation of Rules:
The formation of Prevention and Control of Water Pollution Act in 1974 has helped to a certain degree to prevent water pollution.
Still, adequate measures have to be adopted by every states to ensure that:
(a) Disposal of sewage and industrial wastes has been undertaken properly,
(b) Abuse of water resources to be prevented and
(c) Punishment of erring industries which do not install effluent devices in their factories through penalties and banning has to be undertaken.