In this article we will discuss about:- 1. Occurrence and Distribution of Selaginella 2. Sporophyte of Selaginella 3. Gametophyte 4. Phylogeny.
Occurrence and Distribution of Selaginella:
Selaginella is commonly called the spike moss. It includes over seven hundred species and reported practically from all parts of the world. Majority of the species occur in tropical rain forests and form a characteristic undergrowth on the ground in humid shady habitats. The temperate species grow on the shady sides of the hills.
A few members are xerophytic e.g. S. lepidophylla and S. rupestris. These grow on dry rocky cliffs or on soils that are sandy and periodically become dry. Some of the members (e.g S. oregana) are epiphytic.
About seventy species of Selaginella have been reported from India. Some of the common species are S. chrysocaulos, S. pallidissma, S. rupestris, S. kraussiana etc. Panigrahi and Chowdhuri (1962) have done considerable amount of work on the distribution of Selaginella in India.
Sporophyte of Selaginella:
Habit:
Species of Selaginella are diverse in their habit. The plant body may be creeping (S. kraussiana Fig.59) Sub erect (S. trachyphylla) or erect (S. erythropus). S. alligaus is a climber. In size also there is a great amount of variation ranging from few centimeters (S. selaginoides) to several meters (S. willdenovii, S. pentagona etc.)
Some xerophytic species are capable of withstanding extreme periods of drought. During unfavourable season they role up into a ball and open up during favourable season into a normal plant. These are called resurrection plants.
Classification:
Hieronymous (1902) classified the genus into two sections namely Hameophyllum and Heterophyllum. The section Homeophyllum includes over fifty species (S. rupestris, S. pygmaea, S. uliginosa etc.) and has isophyllous leaves. The stems are monostelic.
There are two subsections in Homeophyllum viz., Cylindrostachya (S. spinosa) and Tetragonostachya (S. oregana). In the section Heterophyllum (S.kraussiana, S. lepidophylla, S. martensi etc.) the leaves are dimorphic and are arranged in four rows on the stem.
Nasu and Seto (1980) have classified the genus Selaginella into four sub genera – Selaginella, Stachygynandrum, Homostachys and Heterostachys based on the spore wall architecture in about 21 species. Jermy (1986) divides Selaginella into five sub genera viz., Selaginella, Ericetorum, Tetragonostachys, Stachygynandrum and Heterostachys.
Morphology of the Plant:
The stem is soft, herbaceous and branched. It is prostrate (heterophyllum) erect or sub-erect (homeophyllum). The branching may be dichotomous or lateral. On the stem are found the leaves which are small measuring a few mm in size. The arrangement of the leaves is spiral in the section Homeophyllum. In Heterophyllum the dimorphic leaves are arranged in four longitudinal rows.
Of these, two rows comprise smaller leaves and opposite to them lie two rows of larger leaves. The leaves are simple and lanceolate to oval in shape. In all the species the leaves on their adaxial surface bear a membranous outgrowth – the ligule. The ligule has a basal region called the glassopodium which is placed in a depression called the ligular pit.
The ligule may be tongue shaped, fan shaped or lobed. A ligule arises from the leaf surface quite early in the ontogeny. The function of the ligule is not well understood. It may be a secretory structure secreting water or mucilage.
Biochemical studies of ligules in seven species of Selaginella have revealed the deposition of Callose. Species growing in shady and moist habitats have less callose than those inhabiting open and dry areas.
Certain curious branches arise at the ramifications of the stem in some species. These are called the rhizophores. These are structures of controversial morphological nature. The number of rhizophores arising at the branching of the stem varies. It may be one (S. kraussiana) or two (S. mariensi).
When there are two rhizophores they have unequal development. The rhizophores develop from meristems called angle meristems present at every branching of the main shoot. In the mode of growth, the rhizophores are positively geotropic and bear tuft of roots at their free end.
Like in other pteridophytes, the root system (in Selaginella) is also adventitious. The roots may arise directly from the stem or may arise from rhizophores. The branching of the root is dichotomous. The reproductive structures generally aggregate at the apices of the branches to form the characteristic strobilus which range from one to five cm.
In some special circumstances (S. patula and S. cuspidata) the strobilar axis may resume growth and produce a vegetative shoot. In S. erythoropus there may be two strobili separated by a sterile region. Selaginella is heterosporous, but the strobilus may or may not have both the types of sporophylls.
Internal Structure:
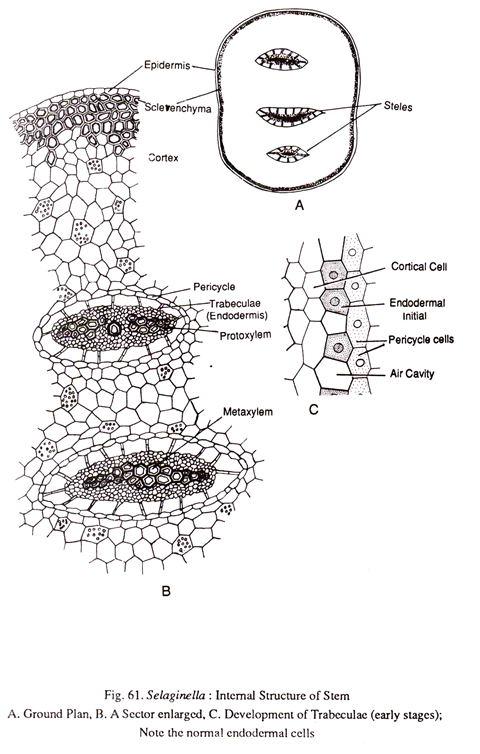
1. Stem:
A transverse section (Fig.61) of the stem shows three main regions namely epidermis, cortex and stele. Epidermis is single layered. The cells are closely packed and there are no stomata.
There is some amount of variation in the composition of cortex. It may be wholly parenchymatous as in the stems of some delicate species (S.flabellate) or partially sclerenchymatous with the hypodermal region is occupied by sclerenchyma (S. kraussiana). In some xerophytic species like S. rupestris and S. lepidophylla the cortex is mostly sclerenchymatous.
The stele is protostelic, it has an outermost endodermis followed by pericycle and vascular tissues. The endodermis in Selaginella is rather peculiar. It is called trabecular because of the radially elongated nature. In some species such as S. rupestris and S. lepidophylla, the endodermis is normal as in other plants. But in a majority of the species the endodermal cells get detached laterally.
At that time a lacuna is formed between the cortex and the stele due to the rapid enlargement of the inner cortical cells than the vascular tissue. The endodermal cells are stretched radially to keep contact between the stele and the cortex. Sometimes these trabeculae (endodermal cells) undergo transverse divisions to form short filaments. In spite of the radial elongation casparian thickenings are clearly noticeable.
The stele shows considerable among of diversity in different species of Selaginella. Usually the stem is traversed by a single stele which is flattened like a ribbon. In some species (S. kraussiana), the stem has more than one independent stele (poly-stele). Sometimes as many as sixteen steles have been recognized running parallelly in the stem. At the region of the branching however all steles converge to give a monostelic appearance.
The xylem is exarch and may be diarch or polyarch (S. spinulosa). In some of the trailing stems of S. spinulosa, the xylem is endarch. In S. laevigata var., lyallii, the stele may show polycyclic solenostelic condition and in some cases the stele may break up into several meristeles.
The xylem mostly consists of tracheids with annular and sclariform thickenings. But some species (S. oregana, S. rupestric, S. densa) are unusual in having vessels. These vessels are of the porous type originated by the absorption of pit membranes in the sclarariformly pitted tracheids.
Though Bruchmann (1897) has recorded a few secondary xylem elements in S. selaginoides, there is no indication of any secondary growth. Surrounding the xylem is the phloem consisting of parenchyma and sieve cells.
Comparative histo-physiological studies conducted on the phloem of Selaginella willdenowii have shown that the seive elements of metaphloem have oblique or transverse end walls. According to Hebant el al (1980) serve pores are found on both lateral as well as end walls and parenchyma cells present between seive elements exhibit enzymatic activities. Surrounding the phloem is the pericycle.
2. Root:
A root in a transverse section (Fig.62) reveals an epidermis, cortex and stele. The epidermis is made up of a single layer of cells. Some of the cells give rise to root hairs. There is variation in the composition of the cortex.
In S. willdenovii, the cortex consists of a few layers of hypodermal sclerenchyma, the rest being parenchymatous. In S. densa the entire cortex is thick walled. It is also possible that in some species the cortex may be wholly parenchymatous.
The stele is protostelic with monarch and exarch xylem. Surrounding the xylem is the phloem. The pericycle is one to three layered. The endodermis may or may not be well defined. In S. densa and some other species there is a well-developed endodermis. The endodermis may or may not show the trabecular nature. According to Webster and Steves (1963), trabeculae of the root are not endodermal, instead they represent the inner cortical cells.
3. Rhizophore:
There is a close similarity between the root and the rhizophore in the anatomy. The rhizophore is always monostelic irrespective of the fact whether the stem is polystelic or monostelic. The stele is always a protostele with some variations in the architecture of xylem. The xylem is usually monarch and exarch.
4. Leaf:
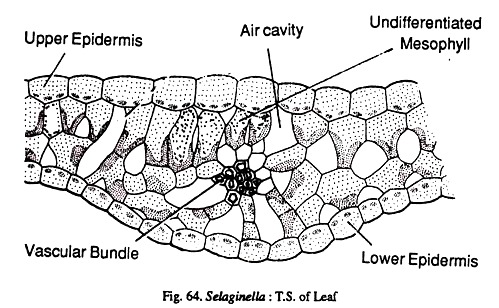
Internally the leaf shows epidermis, mesophyll, and a vascular bundle. The upper and lower epidermis are made up of a single layer of cells. The leaves may be hypostomatic or amphistomatic. The mesophyll is usually undifferentiated being composed of isodiametric cells with intercellular spaces. In S. concinna there is a distinct palisade parenchyma below the upper epidermis.
The mesophyll cells contain a variable number of chloroplasts. In S. willdenovii there are two and in S. martensii there is a single cup shaped chloroplast the chloroplast structure reveals the presence of many pyrenoid like bodies.
This type of chloroplast with a pyrenoid is something unusual and recalls the features seen in Anthocerotales and green algae. The vascular bundle is concentric having a central xylem surrounded by the phloem.
5. Ligule:
Anatomically the- ligule shows many thin walled cells with highly vacuolated protoplasts in the glossopodium. The glossopodium is surrounded by a sheath called the glossopodial sheath.
In S. kraussiana and S. rupestris the sheath cells show casparian strips possibly indicating their endodermal nature. Above the glossopodium, the ligule narrows down and is extended into a long process. The cells at this region are polygonal and filled with dense cytoplasm.
Reproduction:
The sporophyte reproduces by vegetative propagation as well as by spore production.
(1) Vegetative Propagation:
This takes place by the following methods:
(i) Fragmentation:
This is seen in species like S. rupestris which grow under humid conditions. Here the trailing branches of the stem develop adventitious branches, sever their connection from the mother plant and develop into new individuals.
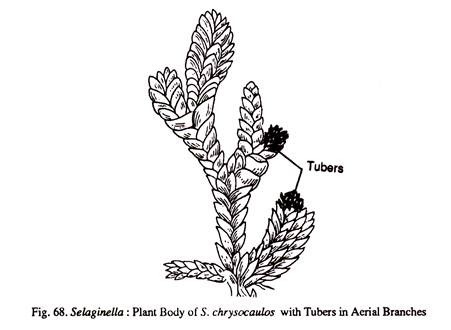
(ii) Tubers:
These may be underground or aerial. In S. chrysorrhizos they are formed at tips of the underground branches that arise from the stem. In S. chrysocaulos the tubers are formed at the tips of ordinary vegetative branches.
(2) Spore Bearing Organs:
The spore bearing organs viz., the sporophylls are aggregated into strobili, at the tips of the branches. Selaginella is heterosporus and usually in the strobili both megasporophylls and micro-sporophylls are formed (S. kraussiana). But in species like S. grassilis each strobilus has only one type of a sporophyll.
Even when the strobilus consists of two types of sporophylls there is considerable variety in their distribution. In S. orgena, one side of the strobilus has micro-sporophylls and the other side megasporophylls. In S. kraussiana there is a single megasporophyll at the base and the rest are micro-sporophylls. In some instances the tip of the strobilus may continue to grow and produce a vegetative shoot (Fig. 69).
Quansah and Thomas (1985) have studied the strobilus morphology in 14 West African and 20 South American species of Selaginella. According to them vertical/oblique projections occur on the adaxial surface of the sporophylls and these are given the name ‘sporophyll-pterix’
A long section of the strobilus (Fig.70) shows a central axis on which are spirally arranged sporophylls. Each sporophyll bears a single stalked sporangium arising in its axil or a little above.
There are two types of sporangia, namely, mega-sporangia and microsporangia. The mega-sporangia have less number of larger spores which develop into female gametophytes, whereas the microsporangia have large number of smaller spores which develop into male gametophytes.
Development of Sporangia:
The early stage of development are similar in both micro- and mega-sporangia. The differentiation into micro- and mega-sporangia starts only at the spore mother cell stage. The sporangial development is of the eusporangiate type. There are however some differences of opinion with regard to the number of cells that go to make up the sporangium.
Lyon (1901) opines that in S. apus and S. rupestris a single superficial cell gives rise to the sporangium. According to Bower (1908), a developing sporangium in a radial section shows only two superficial cells in S. martensii and S. spinulosa. But he says the two cells in reality represent two tangential rows of initial cells.
The sporongial initials arise in the axil of the sporophyll. But it is possible that the initials may arise on the axis a little above the insertion of the sporophyll. The sporangial initials divide periclinally (Fig.73) to form an outer row of jacket initials and an inner row of archesporial cells. The jacket initials divide anticlinally and periclinally to form a two layered jacket.
Meanwhile the archesporial cells divide in all the planes to produce a mass of sporogenous cells. The outermost layer of the sporogenous cells distinguishes itself into tapetum. The tapetum has papilate cells and persists till the maturation of the spores.
Microsporangium:
If a sporangium is destined to become a microsporangium, most of the cells of the original sporogenous mass except for 10-20% survive, undergo reduction division and produce tetrads of haploid microspores.
Mega-sporangium:
If a sporangium is to develop into a mega-sporangium, generally most of the spore mother cells except for one, degenerate. There are however variations in this. In some cases 2-6 megaspore mother cells may function. There is variation in the number of megaspores in a sporangium.
Generally four megaspores are produced by the reduction division of a megaspore mother cell. But in S. monospora there is only one megaspore in the sporangium. An extreme case of 42 spores in is seen in S. willdenovii. In any case the megaspores get nourishment from the tapetum and the degenerating spore mother cells. They gradually enlarge in size and fill the sporangial cavity.
Structure of the Mature Sporangia:
There is colour difference between the microsporangia and mega-sporangia at their maturity. The microsporangium is red, yellow or brown in colour. The mega-sporangium is yellow, orange or chalky white in colour (Fig.74).
The sporangia have a short stalk. The sporangial wall is usually two layered. The outer layer has columnar chlorophyllous cells. The inner layer has thin walled cells. Sometimes a thin tapetal layer is seen persisting in the mature sporangium.
Dehiscence of the Sporangia:
The sporangia break open by the appearance of a vertical slit. The breaking open of the sporangium is effected by the differential hygroscopic response of the wall cells. The vertical split will not reach the base. This basal un-slit portion shrinks and throws out the spores. The force of dehiscence is more in the mega-sporangia so the spores are thrown to a longer distance (6-10 mm) than the microspores (1-1.5 cm). According to Goebel (1881) the microspore masses are ejected in several installments.
Scanning electron microscopic studies of microsporangia of Selaginella (series Articulatae) have shown the presence of annuloid cells marking the area of dehiscence. These cells slowly uncoil (not suddenly as in fern sporangia) helping in the liberation of the spore mass.
Gametophyte of Selaginella:
The spores are of two types namely microspores and megaspores. The former develop into male gametophyte while the latter develop into female gametophyte.
Structure of the Microspore and Development of the Male Gametophyte:
The microspores are very minute in size and range in diameter from 0.015 to 0.05 mm. Soon after separation from the tetrad they will be triradiate but gradually assume a sub-spherical shape. The spore wall is two-layered. The outer exine (exospore) is very thick and is sculptured. The inner inline (endospore) is thin and delicate. The spore consists of reserve food material in the form of oil globules and nitrogenous material.
In a comparative study of spore wall ornamentation Knox (1950), has reported the following six types in various species of Selaginella:
(i) Granulose – S. serpens
(ii) Tuberculate – S. chrysocaulos
(iii) Vercuate – S. mongolica
(iv) Baculate – 5. haemotodes
(v) Saccate – S. rupestris
(vi) Spinosc – S. galcotti
The spore germinates and the development of the male gametophyte begins even before the sporangium dehisces. Generally the male gametophyte develops in situ up to the thirteen celled stage. So when the spore liberation takes place actually it is the liberation of incompletely developed male gametophyte.
The details of the development have been worked out in S. kraussiana. The first division results in the formation of small lenticular cell, the prothalical cell and a larger cell the antheridial cell. The prothallical cell does not divide further. It is the sole representative of the entire vegetative tissue of the male gametophyte. The antheridial cell divides vertically to form the two primary cells of the anthcridium. At this stage there are three cells in the gametophyte (Fig.76b).
The primary antheridial cells next divide by means of a curved transverse wall, as a result there will be four antheridial cells (Fig.76c). So at this stage there are five cells in the gametophyte.
Of the quadrant of cells, the lower two do not divide and they constitute the cells of the jackets layer of the anthcridium. The upper two cells divide by means of a curving vertical wall to form two smaller distal cells and two larger cells. The two smaller cells do not divide further and develop into the jacket cells. At this stage the gametophyte has seven cells (Fig.76d).
The two larger cells divide again by means of a curving wall and the gametophyte now consists of nine cells. Of these nine cells, there will be one prothallial cell, four jacket cells and four antheridial cells. The four antheridial cells undergo periclinal divisions to form a central group of four cells surrounded by eight peripheral cells (Fig.76f).
At this stage the gametophyte consists of thirteen cells (one prothallial cell, four primary androgonial cells and four jacket cells). The peripheral cells are the jacket cells and the central group of four cells constitutes the primary androgonial cells.
At this stage, the micro-gametophyte is liberated from the sporangium. It should be pointed out, however, that the entire development of the male gametophyte is limited to the confines of the spore. Thus the development is said to be endosporic.
Further development in the gametophyte takes place after their liberation. The microspores may fall on the soil to continue the development or may fall into the clefts of the partially opened mega-sporangia where in the vicinity of the female gametophyte the antherozoids are produced. Such a transfer of microspores to the vicinity of female gametophyte, remotely resembles the process of pollination seen in higher plants and may be called incipient pollination.
The four primary androgonial cells divide several times to form a total number of 256 androcytcs, each of which metamorphoses into an antherozoid. The antherozoids are spirally coiled and biflagellate. At this stage the jacket breaks open and the antherozoids come out in a mass.
Structure of the Megaspore and the Development of the Female Gametophyte:
The megaspores are much bigger in size than the microspores and range in diameter from 1.5-5 mm. When they are in tetrad the spores have a triadiate shape but become sub-spherical on separation. The wall of the megaspore is very thick and consists of a sculptured exine, a middle mesospore and a thin intine.
The cytoplasm consists of reserve food in the form of oil globules and nitrogenous material. The amount of nitrogenous material present is considerably less in comparison with the microspore. Chemical analysis of the stored food in megaspores of Selaginella reveals that they have 48% fats, 0.43% nitrogenous matter and 1.26% mineral material.
As in the male gametophyte, even here the early development takes place in situ but the stage at which the mega-gametophyte is liberated from the sporangium varies in different species. In S. kraussiana liberation takes place after the formation of archegonia in the gametophyte. In S. rupestris even fertilisation takes place in situ. In S. spinulose the development is initiated only after liberation.
In a megaspore which is about to germinate, the exine expands much faster than the mesospore so that the two get separated except at the apical region. It is believed that this space is filled with fluid containing plastic materials.
The first sign of germination is the division of the megaspore nucleus. There is no wall formation. The free nuclear divisions continue, to form a large number of nuclei. To start with, the nuclei are arranged in the peripheral cytoplasm with the central region being occupied by the vacuole (Fig.77a).
Gradually the peripheral cytoplasmic layer extends to the centre so that vacuole gets obliterated. At this stage there is more concentration of the nuclei and cytoplasm at the apical region than in the basal region. It is in this apical region that cell wall formation begins. To start with, a single layer of regular hexagonal, uninucleate cells is formed. Some of the cells at the margin are irregular and may have two or more nuclei.
The cells formed are initially open on the lower side, hence are known as areoles. With the continued wall formation at this region, an apical cellular cushion is formed. This cushion is three layered thick towards the middle but one layered thick towards the margin (Fig.77b). The lowermost cells of the apical cushion have a much thickened lower-wall constituting the diaphragm.
The diaphragm separates the apical cushion from the lower free nuclear portion. Even in this portion cell wall formation sets in either at the time of development of archegonia or during the formation of embryo. Ultimately the entire mega-gametophyte becomes cellular and occupies the whole of the interior of the spore cavity.
The cells in the apical region are polygonal and uinculeate. But the cells of the basal region are irregular in shape and may be multinucleate. These cells are rich in reserve food and provide nutrition for the developing embryo.
Development of the Archegonium:
All of the superficial cells of the apical cushion are potentially archegoniate, however only a few archegonia are produced. The archegonial initial divides periclinally to form a lower central cell and a primary cover cell (Fig.77f). The central cell is separated from the diaphragm only by a single layer of cells. The primary cover cell divides twice anticlinally where the second division is at right angles to the first one to form four neck initials.
Transverse divisions in the neck initials form a short, two celled high neck. Meanwhile the central cell divides transversely to form a primary canal cell and a primary venter cell. The former docs not divide further and forms a neck canal cell while the latter divides once transversely to form a venter canal cell and an egg cell.
Liberation of megaspores:
As in the male gametophyte even here the female gametophytes are confined to the spore interior. The liberation of the megaspores enclosing the female gametophytes usually takes place after the formation of the archegonia.
On falling upon a suitable substratum, the exine of the spore ruptures exposing the apical cushion of the gametophyte. The apical cushion protrudes out a little and some of its cells produce rhizoids. These rhizoids anchor (he gametophyte to the substratum and retain water necessary for fertilization.
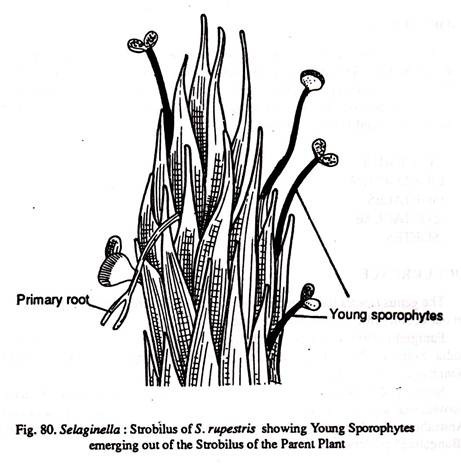
Lyon (1901) reports that in Selaginella rupestris the megaspores are retained permanently in the mega-sporangium, develop into gametophytes and undergo fertilization. Even embryogeny and emergence of young sporophytes take place in situ indicating a viviparous condition. (Fig.80)
Fertilization:
The mature archegonia are embedded in the gametophytic tissue. The ventral canal cell and the neck canal cell degenerate leaving only the egg. Fertilization may take place when the mega-gametophytes are on the soil or when they are still within the mega-sporangia. The antherozoids swim in a thin film of moisture and fertilize the egg.
Embryogeny:
The first division of the zygote is transverse. This results in the formation of an upper epibasal cell and a lower hypo-basal cell. The entire epibasal cell develops into a suspensor. The hypo-basal cell contributes to all the parts of the embryo. Embryogeny is thus said to be endoscopic.
Further development varies in different species. In S. martensii, the hypo-basal cell gives rise to stem apex, cotyledon, foot and root. The epibasal cell forms several cells all of which form a long suspensor pushing the developing embryo deep into the gametophyte. The hypo-basal cell divides vertically followed by another vertical division to form a quadrant of cells.
Of these, one divides by means of an oblique wall to form the apical cell. Further divisions are irregular and result in the formation of a mass of cells. The cells that are nearer to the suspensor actively divide and form the foot. Repeated divisions in the foot region twist the embryo at right angles to the suspensor.
The remaining cells differentiate into the cotyledons. The cotyledons immediately develop a ligule. Meanwhile the shoot apical cell would have divided to form the shoot apex. Development of the root initial takes place quite late in embryogeny. The root initial differentiates between the foot and the suspensor. With the formation of the root the young sporophyte becomes independent.
The stem for some time remains un-branched but very soon branching takes place. In S. kraussiana the suspensor is much reduced and is replaced by the embryo tube (embryosclauch) which is nothing but the gametophytic tissue developing from the cells surrounding the archegonium.
Apogamy and parthenogenesis:
In some cases, the archegonial initials develop directly into an embryo. Parthenogenesis i.e., the direct development of egg without fertilization, has been reported in S. intermedia.
Chromosome Numbers:
Manton (1950) has recorded n = 9 as the basic chromosome number. Wang el al (1984) have reported the chromosome number to be n = 8 in S. labordu and S. omeinensis. Loyal and Kumar (1985) have demonstrated the following chromosome numbers
S. chrysocalos r n = 12 (2n = 24)
S. subdiaphana – n = 8 (2n =16)
S. pallida – n = 9 (2n = 18).
Morphology of the Rhizophore:
Three different views have been expressed to account for the morphological nature of the rhizophore:
(1) They are capless roots;
(2) They are leafless shoots;
(3) They are organs sui generis.
The first view is supported by Van tieghem, Harvey Gibson, Uphoff etc.
The root like characters of rhizophore are as follows:
(a) They are positively geotropic,
(b) They are leafless
(c) The rhizophores are always monostelic even in polystelic stems.
According to Wochok and Sussex (1974) auxins are transported acropetally (it is basipetal in stems) in rhizophores confirming their root nature. They used labelled IAA to prove their point. The second view is supported by Pfeffer, Treub, Bruchmann, Velenovsky etc.
The shoot like characters of the rhizophore are:
(a) Absence of root caps and root hairs,
(b) Their exogenous origin and
(c) Their development from angle meristems present between the two branches of the stem.
Some of the experimental evidences also seem to indicate that the rhizophores are capable of developing into leafy shoots under controlled conditions. The experiments conducted by Williams (1938) in S. martensii have shown that if the normal shoot apex is removed, the nearest rhizophore develops into a leafy shoot. Ontogenetical studies conducted by Cusick (1954) in S. willdenoii have shown that the angle meristem is a part of the embryo shoot.
The third view is supported by Goebel, Bower etc., who regard that the rhizophore is neither a shoot nor a root but an organ of its own kind.
According to Schoute (1909), the rhizophore cannot be regarded as an organ sui generis because it shares many characters with the root and stem; while an organ sui generis has to be a novelty and should not show any resemblance to the existing organs. With these controversies the morphology of rhizophore remains an open question, though in all probability it is closer to the stem than to the root
Phylogeny of Selaginella:
Selaginella is a highly interesting and a highly specialized genus showing much diversity in the structural features. Selaginella shares many characters with Lycopodium. The general plan of the plant body is essentially same in both the members. The leaves are microphyllous in both, though they are ligulate in Selaginella. The biflagellate nature of an therozoid is another character that is shared between Selaginella and Lycopodium.
Selaginella has some unique characters not seen in any other lycopsids. These are the rhizophores, presence of vessels etc. Heterosporous condition leading to the formation of seed habit is very well exhibited in Selaginella.
The partial retention of the mega-gametophyte (sometimes even up to fertilization) clearly indicates one of the living examples in the evolution of a seed habit. The ultimate liberation of the mega-gametophyte, poor development of the mega-sporangial wall not reaching the stage of an integument and more number of spores (though rarely only one spore is functional) in the sporangium are some of the characters which have tied down Selaginella to the group Lycopsida of pteridophytes, otherwise it had all the attributes to qualify as a Spermatophyte.