In this article we will discuss about:- 1. Occurrence and Distribution of Ophioglossum 2. Sporophyte of Ophioglossum 3. Gametophyte 4. Phylogeny.
Occurrence and Distribution of Ophioglossum:
Of all the genera of the family, Ophioglossum is the most widely distributed being found in almost all the parts of the globe.
The genus is divided into three sub-genera viz.:
(i) Euophioglossum e.g., O. moluccanum, O. vulgatum
(ii) Ophioderma e.g. O. pendulum etc., and
(iii) Cheiroglossa e.g., O. palmatum.
The number of species in the genus varies. Most of the pteridologists include 28 species in the genus. About twelve species are found in India. Some of the common Indian species are O. vulgatum, O. pendulum, and O. ailchisoni, O. vulgatum is known to occur in Nandi hills (Bangalore District).
Khandelwal and Goswami (1984) have reported the occurrence of a new species from India (Ophioglossum eliminatum), while Bir and Bhusri (1985) have reported the occurrence of ophioglossaceae (Ophioglossum and Botrychium) in Shimla Hills. Sharma and Singh (1985) have described the morphology and anatomy of seven species of Ophioglossum from India (O. reticulatum, O. petiolatum, O. nudicaue etc.).
Ophioglossum generally grows in humus rich, shady, moist soil. Some of the species (O.pendulum) are epiphytic growing in humus packets of trees. O. palmatum grows on rotting tree trunks suggesting a saprophytic nature.
Sporophyte of Ophioglossum:
Morphology of the plant:
In most of the species, the plant body possesses a short, erect, tuberous rhizome exhibiting radial symmetry. In O. pendulum, O. intermedium the rhizome is dorsiventral. The branching of the rhizome is infrequent, may be dichotomous or lateral.
The upper surface of the rhizome produces leaves in an irregular species, the number of leaves produced per season varies. In temperate species only one leaf is formed per growing season.
In tropical species however, the number may be 4-5 per year. The growth of the leaf is extremely slow. A leaf primordium is underground for the first three years and only in the fourth year it comes above the ground. The leaves may be erect or pendant as in 0. pendulum.
A great amount of diversity is exhibited in the nature of the leaves. Generally a leaf has two parts viz., petiole and lamina. The petiole is erect and long. It bears an expanded, simple and entire lamina. At the junction of the lamina and the petiole arises the fertile spike the reproductive structure of the plant.
In O. pendulum the distinction between the lamina and the petiole is lost. Here the leaf is long (1-1.5 metres) and dichotomously branched. In O. palmatum the petiole bears at its tip a number of palmate lobes of the lamina. Below the lamina there are two rows of fertile spikes.
The lamina has a reticulate venation but it is not closed. Bhambie and Prakash (1982) have reported that the reticulate venation of Ophioglossum is in contrast with the open dichotomous venation seen in Botrychium and Helminthn achys. The veinlets end blindly in the mesophyll (Fig.122). This is very characteristic of Ophioglossum. There is no distinct midrib. At the base of the petiole are found two stipules (forming a sheath) enclosing the young leaf of the next season.
The leaves of Ophioglossum do not exhibit circinate vernation that is so typical to ferns.
The rhizome is anchored to the soil with the help of numerous adventitious roots that are produced from its (rhizome) under surface. There are no root hairs.
The roots may be unbranchcd (Euophioglossum), infrequently branched (Cheiroglossa) or frequently branched (Ophioderma).
Usually only one root is formed at the base of the leaf of the current season. Unlike leaves, the roots are perennial. The roots have a mycorrhizal association which compensates the absence of roots hairs.
Internal Structure:
1. Rhizome:
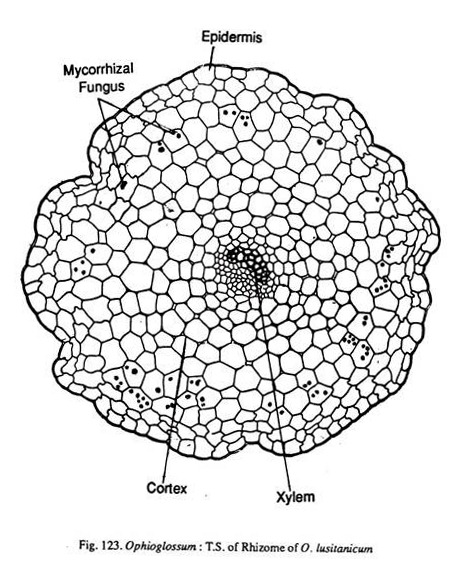
A transverse section of the rhizome shows an epidermis, cortex and the central stele. The outline of the section is irregular and discontinuous due to the presence of roots and leaf bases. The epidermis is single layered. Cortex is wholly parenchymatous. The cells are loosely arranged with large number of intercellular spaces.
In older rhizomes epidermis is replaced by a periderm layer, but there is no cork cambium. According to Campbell (1939), the periderm belongs to the old leaf bases. The presence of endodermis is doubtful in older rhizomes. In younger rhizomes an endodermis has been identified (O. pendulum). According to Lang (1922), there may be an internal as well as an external endodermis. Pericycle may be present.
The stelar organisation is variable. In O. reticulatum the basal region of the rhizome is protostelic which becomes solenostelic as it travels up. In a majority of the species the stele is an ectophloic solenostele. Occasionally leaf gaps may overlap resulting in a dictyostelic condition (Fig. 120). Sometimes in the same rhizome at different regions the stele ranges from protostele to a dictyostele.
Each merisiele is semi-lunar in shape with the concave side directed towards the center. Xylem is endarch. In O. palmatum the protoxylem elements are not well defined. Phloem is collaterally arranged with reference to xylem. Generally phloem consists of four to five layers of cells. But in O. pendulum pholem is single layered and is separated from xylem by a layer of parenchyma cells.
Boodle (1899) has recorded a small amount of secondary thickening in the rhizome of O. vulgatum. The pith is composed of thin walled parenchyma. Mixed pith has been reported in O. pendulum and O. aitchisoni where tracheids are intermixed with parenchyma cells. Anatomical studies in the family ophioglossaceae by Prakash and Bhambie (1980) have shown that true vessels are absent in Ophioglossum.
2. Leaf:
From the rhizome a single leaf trace is given off; but at the base of the petiole it divides into two strands and enters the leaf. But in O. palmatum and several other species each leaf gap is associated with two leaf traces. The leaf traces, further up in the petiole divide into a number of vascular bundles and are arranged in a ‘C’ shaped outline with the opening directed towards the adaxial surface.
The bundles are collateral with endarch xylem. Phloem is on the outer surface of the xylem and is separated from the latter by a layer of parenchyma. There is no bundle sheath. Surrounding the vascular bundles is a ground tissue composed of parenchyma, a few outer layers of which have chloroplasts. The ground tissue is protected by a single layered thick walled epidermis.
The lamina on either side is bounded by a single layered epidermis. Leaves are amphistomatic, but the number of stomata in the upper and lower epidermis varies. In O. vulgatum they are equal in number on both the surfaces; in others (O. reticulatum) stomata are more in number towards the lower epidermis.
Stomatal studies in the leaves of Ophioglossum reticulatum have shown that they are amphistomatic with anomocytic and diacytic type of stomata. The mesophyll is undifferentiated (Fig. 122) and is composed of thin walled isodiametric chlorophyllous cells. The mesophyll consists of a number of veins (bundles) whose structure is similar to those of the petiole.
3. Root:
A root in a transection shows a single layered epidermis, a massive parenchymatous cortex and a central stele (Fig. 123). The epidermis is short lived and is replaced by exodermis (O.fibrosum), or it may persist with suberization. The cortex though wholly parenchymatous is divisible into two zones.
In the outer zone, the cells are angular and closely packed without any intercellular spaces. It is in this zone that the fungal hyphac are present. The hyphae are aseptate. The inner zone of the cortex has loosely arranged parenchyma cells with no mycorrhizal fungus.
The stele of the root may be mono, di- tri tetra or even pentarch. But the first two conditions are more frequent. In diarch roots (Fig. 123), the xylem is plate like with a protoxylem point at each end of the plate. In triarch stele, the xylem groups are separated by parenchyma.
Reproduction:
As usual there are two methods of reproduction, Vegetative propagation is brought about by the formation of adventitious buds (root tubers), e.g., O. pendulum, O. vulgatum etc.
Spore Producing Organs:
The spore producing organ is generally called a ‘fertile spike’. But Campbell (1939) calls it ‘sporangiophore’. The fertile spike is very much different from the strobili of Lycopodium or Selaginella in that the sporangia are completely embedded and there is no trace of any sporophyll. There is a central axis like structure and on either side of this are found two rows of sporangia, arranged paraded to the long axis of the fertile spike.
The number of sporangia per fertile spike varies from 6 to 20. Many vascular strands run into the fertile spike and they frequency anastomose. The lateral strands that arise from these main strands travel in between the sporangia, take 90° turn and reach the base of the sporangia.
Development of the fertile spike and Sporangium:
Our knowledge of the development of the spore producing organs is mainly due to the work of Bower (1896). A fertile spike arises as a conical hump of meristematic tissue on the adaxial surface very early in the ontogeny of the leaf.
Further growth of the fertile spike is brought about by a pyrimidal apical cell with four cutting faces. These four faces cut off cells regularly; as a result the young fertile spike consists of four quadrants. Of these, two are oriented perpendicular to the lamina, while the other two are parallel to it (Lamina).
As growth proceeds in the fertile spike a strip or band of cells gets differentiated in the epidermal layer of the two quadrants in the plane parallel to the lamina. Each strip is 2 to 3 cells broad and many cells thick. These strips of cells are called ‘sporangiogenic bands’ (Fig. 124a), because all the sporangia are derived from these.
The sporangiogenic bands (they are two in number) increase their extent by periclinal and anticlinal divisions. Early in the development, all the cells in the sporangiogenic band are alike. Very soon certain block of cells in the hypodermal region differentiate themselves from the remainder by their dense cytoplasmic contents and prominent nuclei.
These constitute the archesporial cells (Fig. 124c). A little later, the hypodermal region comes to consist of (in two rows) alternate groups of fertile and sterile cells, each fertile area marking the position of a future sporangium.
The development of the sporangium is no doubt of the eusporangiate type. But it is different from others in having a common group of mother cells giving rise to all the sporangia. It may be assumed that all the cells of the sporangiogenic band are potentially sporogenous and due to the process of sterlization, the band breaks up into a number of archcsporial masses.
The cells of the sporangiogenic band external to each archcsporial mass divide anticlinally and periclinally, to form the multilayered wall of the sporangium. At this stage the sporangia project as hemispherical bulges from the surface of the fertile spike.
A tapctum differentiates itself surrounding the sporogenous mass; but its origin is controversial. While Bower (1911) argues that the tapetum is derived from the outer sporogenous tissues,. Burlingame (1907) believes that it is derived from the inner layers of the wall.
Whatever may be the origin, the tapetum consists of many layers, whose cells disintegrate at the time of reduction division of the spore mother cells forming a plasmodial mass. The spore mother cells divide meiotically and produce haploid spores. All the spores are of the same type (homosporous). The spore output is enormous; it may be 10,000 or more per sporangium.
Dehiscence of the Sporangia:
There is no special dehiscence mechanism. The sporangia open by a transverse slit, where rows of thin walled cells would have been differentiated. As Smith (1955) opines dehiscence seems to be the result from a drying out and shrinking of the sterile tissues within the spike. The spores are minute, very light and wind disseminated.
Gametophyte of Ophioglossum:
Structure and Germination of Spores:
Spores are tetrahedral in shape and have a two layered wall. The outer layer (exine) is sculptured with many pits. The cytoplasm of the spore is rich in reserve food, specially fat. Spore germination is immediate. It may be delayed sometimes from a month to a year depending on the species. The spore absorbs moisture, swells and the exine ruptures protruding a papilla like germ tube. First division in the spore is transverse and of the resultant cells, the lower divides vertically.
It is believed that further development beyond this three celled stage proceeds only when there is an infection by the mycorrhizal fungus. The fungus may provide growth stimulants necessary for the development of the gametophyte. Further cell divisions are irregular and it is difficult to trace them.
Structure of the Mature Pro-thallus:
The gametophyte of Ophioglossum like that of Psilotaceae and most of the species of Lycopodium, is a subterranean structure without any chlorophyllous tissue. The shape of the pro-thallus varies in different species. In O. moluccanum it is cylindrical and less than one centimetre in length. There is a slightly tuberous (Fig. 125) region at the base. In O. pendulum the gametophyte is circular and has radiating branches.
The pro-thallus has only parenchyma. The tuberous basal region consists of the mycorrhizal fungus. Prothalli may be annual (O. moluccanum) or perennial (O. pendulum) Rhizoids may or may not be present.
Reproduction:
There does not seem to be any vegetative propagation in the gametophyte. Sexual reproduction is by the formation of antheridia and archegonia. The prothalli are monoecious. Sex organs are scattered all over the pro-thallus.
Development and Structure of the Antheridium:
Nozu (1961) has studied the antheridial development in Ophioglossum. The antheridium develops from a superficial initial cell. This divides periclinarlly to form an outer jacket initial and an inner primary androgonial cell. According to Campbell (1911) the jacket initial first divides anticlinally (Fig.126a) to form two daughter cells.
Further, by an intersecting wall a triangular cell is differentiated in the jacket. This functions as an apical cell and cuts off segments on all the three sides. After a few divisions, the apical cell transforms itself into an opercular cell.
The primary andorogonial cell divides and re-divides to form a mass of androcytcs (Fig.126b, 126c) which metamorphose into antherozoids. A mature antheridium has a single layered jacket enclosing a mass of antherozoids, which are coiled and multi-ciliate. At maturity the opercular cell in the antheridial jacket disintegrates releasing the antherozoids.
Development and Structure of the Archegonium:
A superficial cell functioning as the archegonial initial divides periclinally and forms an upper primary cover cell and a lower cell (Fig. 127a). The lower cell divides again to form a basal cell and a central cell (Fig.127b).
The primary cover cell forms a neck 3-4 cells in height. The basal cell docs not contribute to any part of the archegoniuim. The central cell divides, and its derivatives develop into a neck canal cell (with two nucleus) a venter canal cell and an egg cell.
In a mature archegonium (Fig. 127f) the neck projects out a little. Fertilisation is as seen in other pteridophyts.
Embryogeny:
There seems to be a great variety in the early embryogeny in different species of Ophioglossum. The first division of the zygote is usually transverse, embryogeny is exoscopic and no suspensor is formed. The two cells (cpibasal and hypo basal cells) next divide vertically resulting in the formation of a quadrant. Further divisions seem to be irregular. The differentiation of the primary parts of the embryo takes place rather late.
In O. vulgatum, one of the hypo basal quadrant gives rise to the foot and the other to the root. Stem apex is differentiated from the epibasal region at a very late stage.
In O. moluccanum the epibasal half gives rise to cotyledon and probably to root also. Hypo basal region forms a massive foot.
In O. pendulum the hypo basal region forms the foot, while the epibasal region forms the root.
An apical cell is distinguished in the root and as a result of this, the root grows out of the gametophyte. Later a bud arises on the root which develops into the adult sporophyte. Growth is extremely slow and it may be 8-10 years before the first green leaf appears above ground.
Chromosome Number:
There is no uniformity in the basic number O. pusitanicum has n = 125 – 130. In O. vulgatum n = 250 – 260. The highest number recorded is in O. reticulatum where n = 631 + 10 fragments.
Morphology of the Fertile Spike:
There are several interpretations concerning the morphological nature of the fertile spike. Bower (1896) equated the fertile spike to a long septate sporangium arising on the sporophyll. The origin of sporangia from a common mass of mother cells is an evidence to this view. But later Bower himself abandoned this hypothesis.
It is now generally agreed upon that the fertile spike is pinna like. According to Goebel (1915) the fertile spike represents a single modified pinna.
According to Roeper (1859) the fertile spike should be equated with two pinnae which have fused laterally. According to him there are three pinnae in the leaf. Of these, the lateral two are fertile and fuse to form the fertile spike while the terminal sterile one forms the lamina.
Chrysler (1910) and Bower (1926) support Roeper’s views. A convincing evidence for this two pinnae theory is provided by the vascular traces. The fertile spike has a vasculature that would go to a pair a pinnae.
According to Zimmermann (1930), the fertile spike is a modified dichotomy of a shoot. Originally, as Zimmermann (1930), holds there were two dichotomies, of which one limb became photosynthetic and the other fertile. Khandelwal (1986) critically investigated the fertile spike in 12 species of Ophioglossum and opined that “the fertile spike is derived from the modified pinnae of a compound leaf”.
Phylogeny of Ophioglossum:
There can be no doubt that Ophioglossaceae represent a primitive stock of the fern flora. The presence of fertile spike though unique in Ophioglossum may be said to be something intermediate between a strobilus and a sorus. The fact that it is often called a sporangiophore clearly illustrates that it has not altogether lost the strobilar nature.
Among the three genera of the family, Ophioglossum seems to be the most primitive having resemblance to the ancestors of the ferns. Bower (1896) disagrees with this and holds that Ophioglossum represents an advancement over the other two genera.
The simplicity in Ophioglossum, according to Bower is a derived condition. Though the Ophioglossaceae represent a primitive fern stock, as a group, no doubt, they are specialised to a high degree.
Marattiales:
The Marattiales include a primitive and compact group of ferns in which the sporangia are borne on the abaxial (away from the axis) surface of the leaf. Sporangia are grouped into sorus. Sometimes sporangia are fused laterally to form a synangium. Leaves have a pair of stipules at the base of the petiole, which is not seen in the other groups of ferns. All the members are homosporous.
The order includes one family Marattiaceae (Angiopteridaceae) with seven genera viz., Angiopteris, Marattia, Archangiopteris, Macroglossum., Protomarattia, Danaea and Christensenia (Kaulfussia) . Of these Angiopteris is discussed here.