This article throws light upon the top four types of anthocyanins . The four types are: 1. Cyanidin 2. Malvidin 3. Delphinidin 4. Peonidin.
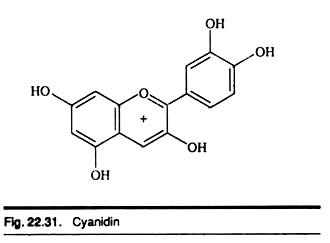
Anthocyanins: Type # 1. Cyanidin:
Cyanidin is a natural organic compound which is classified as a flavonoid and an anthocyanin.
Sources:
Grapes, bilberry, blackberry, blueberry, cherry, cranberry, elderberry, hawthorn, loganberry, raspberry, apples, plums, red cabbage, the highest concentrations of cyanidin are found in the skin of the fruit, and biosynthesis of cyanidin 3-O-glucoside in Escherichia coli was demonstrated.
Uses:
i. Antioxidant effects which protect cells from oxidative damage and reduces the risk of heart diseases and cancer.
ii. Cyanidin may aid in preventing obesity and diabetes
iii. Anti-inflammatory effects
iv. Important future role in cancer therapy
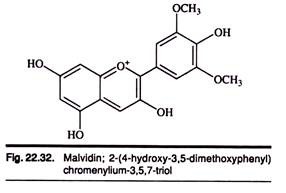
Anthocyanins: Type # 2. Malvidin:
Malvidin is an anthocyanidin. As a primary plant pigment, its glycosides are highly abundant in nature. Slightly acidic and neutral solutions of Maldivian are characteristically of a red color, while basic solutions of Maldivian yield a blue color.
Sources:
It is primarily responsible for the color of red wine, Vitis vinifera being one of its sources. It is also one of the anthocyanidins responsible for the blue pigment found in the Primula polyanthus plant.
Anthocyanins: Type # 3. Delphinidin:
Delphinidin is an anthocyanidin and a primary plant pigment. Delphinidin gives blue hues to flowers like violas and delphiniums. It also gives the blue-red color of the grape and can be found in cranberries and concord grapes. Delphinidin, like nearly all other anthocyanidins is pH sensitive, and changes from blue in basic solution to red in acidic solution.
Anthocyanins: Type # 4. Peonidin:
Peonidin is an anthocyanidin and a primary plant pigment. Peonidin gives purplish-red hues to flowers such as the peony, from which it takes its name, and roses. It is also present in some blue flowers, such as the morning glory.
Like most anthocyanidins it is pH sensitive, and changes from red to blue as pH rises. This happens because anthocynaidins are highly conjugated chromophores. When the pH is changed, the extent of the conjugation (of the double bonds) is altered, which alters the wavelength of light energy absorbed by the molecule.
At pH 2.0, peonidin is cherry red; at 3.0 a strong yellowish pink; at 5.0 it is grape red-purple; and at 8.0 it becomes deep blue; unlike many anthocyanidins, however, it is stable at higher pH, and has in fact been isolated as a blue colorant from the brilliant “Heavenly Blue” morning glory (Ipomoea tricolor Cav cv).
A very large question, however, has been raised about anthocyanidins’ penetration and retention in human cells in vivo, due to their rapid elimination from the human body.
Use:
Peonidin, like many anthodcyanidins, has show potent inhibitory and apoptotic effects on cancer cells in vitro, notably metastatic human breast cancer cells.
Source:
i. Cranberries, Blueberries, plums, grapes, and cherries also contain significant amounts, ranging from 5 to 12 mg/100g.
ii. Only fresh fruit has been shown to contain significant peonidin.
iii. It has also been isolated from raw black rice and black bananas.