In this article we will discuss about:- 1. Introduction to Respiratory Mechanism in Plants 2. Kinds of Respiration in Plants 3. Working of ETS (Electron Transport System).
Introduction to Respiratory Mechanism in Plants:
In higher plants air enters the leaves and young stems by the stomata and through the lenticels in older stems in which secondary growth has taken place. Once within the plant the air is distributed through the intercellular spaces from where it is picked up by the cells and used for respiration.
Therefore, it becomes clear that the respiratory mechanism in plants is different from that of animals in one very important aspect that it does not involve breathing movements. For these reasons, respiration is plant is defined in strictly scientific manner as the process of oxidation of food to obtain energy. For the oxidation, oxygen need not be utilised always and even carbon dioxide may not be released in some cases.
Respiratory Quotient:
Respiratory quotient (RQ) or respiratory ratio is defined as the ratio of the volume of CO2 released to the volume of O2 consumed in respiration per unit time.
The measurement of RQ gives a clue about the type of food being oxidised by the cell at a given time.
The RQ value of carbohydrates is one, for proteins and lipids less than one, for organic acids more than one, for succulents it is zero and for anaerobic respiration it is infinite.
Photorespiration:
Krotkov (1963) introduced the term photorespiration. It is defined as the process of respiration taking place in green plant, only in the presence of light. During this process-CO2 is evolved but no ATPs are produced. It is normally observed in plants like pea, sunflower, wheat, barley, etc., in which C3-cycle operates. It is absent in tropical grasses (maize, sugarcane, etc.) and in some of the dicots like Amaranthus and Artiplex.
Site of Photorespiration:
It occurs only in green parts of the plant and involves three organelles viz.; chloroplast, mitochondria and peroxisomes.
Kinds of Respiration in Plants:
There are two chief types of respiration:
1. Aerobic Respiration:
This is a general term covering a vast series of chemical reactions of very complicated nature, by means of which the chemical energy of foods is converted into some easily available or ready to use form, usually adenosine triphosphate (ATP) in the presence of oxygen.
The energy contained in the food is made available for work in many ways, but the most common method is via glycolysis and the Krebs cycle. Any food can be used for the production of energy, but glucose, the basic product of photosynthesis is used by most of the living cells for this purpose. Glucose is oxidised and broken in its components, i.e., CO2 and water with the release of energy which is stored in the form of ATP.
The overall reaction for the respiration is:
C6H12O6 +6O2 → 6CO2 + 6H2O + Energy
The series of reaction taking place in the decomposition of glucose are divided in two phases. The Phase I, is known as glycolysis, and takes place in the cytosol of the cell. In this phase neither oxygen is used nor are the CO2 and water produced. Therefore this step is common in anaerobic as well as aerobic respiration.
The second phase is known as Krebs cycle, in which oxygen is utilised, energy is produced with the formation of water and CO2. This phase takes place in the mitochondria and not in cytosol.
Phase I: Glycolysis:
This includes a series of enzymatic chemical reactions which take place in the cytosol using the glucose as the raw material for the production of energy. Glycolytic pathway is also called as Embden-Meyerhof-Parnas (EMP) Pathway after the names of the three German scientists who discovered it.
Energetics of Glycolysis:
Number of ATP molecules consumed:
(i) Glucose → Glucose-6-phosphate = 1 ATP
(ii) Fructose-6-P → Fructose-1, 6-diphosphate = 1 ATP
Number of ATP Molecules Produced:
Due to substrate level phosphorylation:
(i) 1, 3-diPGA → 3-PGA =1 ATP × 2 = 2 ATPs
(ii) PEPA → Pyruvic acid = 1 ATP × 2 = 2 ATPs
(iii) Due to oxidation of NADH2 (Produced in the conversion of 3-PGA into 1, 3-diPGA) in Electron transport system, i.e., Oxidative phosphorylation) = 3 ATP × 2 = 6 ATPs
Total number of ATPs produced = 10 ATPs
Gain of ATPs at substrate level → (4 ATPs – 2ATPs) = 2 ATPs
Gain of ATPs at ETS level (only under aerobic conditions) = (10 ATPs – 2 ATPs) = 8 ATPs
Phase II:
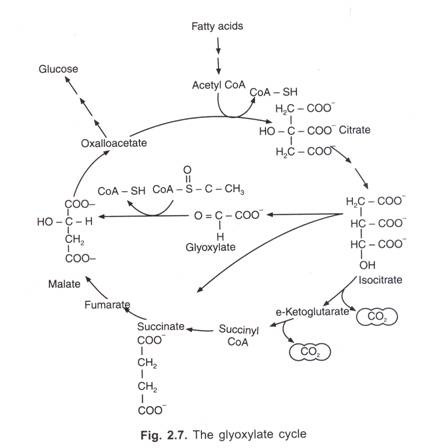
Krebs’ citric acid cycle or tricarboxylic acid cycle : This cycle includes a series of chemical changes through which pyruvic acid is completely broken down to CO2 and H2O with an uptake of oxygen and release of energy, which is stored as ATP Most of the steps of this cycle were worked out by Sir Hans A. Krebs. In recognition of his work Krebs was awarded Nobel Prize in the year 1953. All the reactions are enzyme controlled and take place in a definite order in the mitochondrial matrix. The greater part of the energy of the food is made available in this phase.
For participation of pyruvic acid in the reactions of Krebs cycle, it is first of all converted into acetyl coenzyme A’ by the process of oxidative decarboxylation. This is a very complex process and requires the presence of a multienzyme system called as pyruvic acid dehydrogenase complex. This enzyme complex includes three enzymes (viz. Pyruvic acid decarboxylase, lipoyl transacetylase and lipoyl dehydrogenase) and six co-factors (viz., coenzyme ‘A’, thiamine pyrophosphate, NAD, Lipoic acid, FAD and Mg++-ion).
The acetyl CoA, thus produced, now enters the Krebs cycle. It acts as the connecting link between glycolysis and Krebs cycle. It is also known as the active acetate. It was discovered by Lohman.
Working of ETS (Electron Transport System) in Respiration of Plants:
1. The pairs of hydrogen atoms removed from the substrates in various oxidation steps of glycolysis and Krebs cycle are accepted by NAD or FAD. It results into formation of NADH2 or FADH2.
2. NADH2 is reoxidised by NADH-dehydrogenase. The hydrogen atoms are accepted by its coenzyme FAD which gets reduced to FADH2. The energy released during oxidation of NADH2 is utilised for joining ADP and inorganic phosphate to synthesize one molecule of ATP.
3. FADH2 passes its pair of hydrogen atoms to coenzyme Q which gets reduced into coenzyme QH2 and FADH2 gets reoxidised to FAD.
4. The oxidation of CoQH2 is brought about by the transfer of electrons (2e–) to the ferric iron (Fe+++) of the cytochromes while the protons (2H+) are expelled out in the perimitochondrial space.
5. From CoQ, the electrons are accepted first of all by the Fe of cytochrome ‘b’ from where they pass sequentially through cytochrome ‘C1’ ‘c’ ‘a’ and ‘a3’, causing them to be reduced and oxidised alternately.
Cytochromes are conjugated proteins having haem prosthetic group. The haem group facilitates the passage of electrons by means of its Fe+3 atoms which becomes reversibly reduced and oxidised by accepting and donating electron according to the scheme- ![]()
6. During transfer of electrons from cytochrome ‘b’ to cytochrome ‘c’ and from cytochrome ‘a’ to cytochrome ‘a3‘, one molecule each of ATP is generated by phosphorylation of ATP.
7. The electrons are finally passed on to one atom of oxygen which results into its activation. The activated oxygen reacts with the 2H+ ions (made available from perimitochondrial space) to form water.
In this way oxidation of NADH2 via ETS provides three molecules of ATP. But FADH2 on oxidation by ETS, yields only two ATP molecules.
Energetics of Krebs cycle:
Percent Efficiency of Respiration:
If a molecule of glucose is completely oxidised in air it produces 686,000 calories, while as a result of complete oxidation of glucose molecule inside an aerobically respiring cell, 38 molecules of ATP are produced.
For synthesis of one ATP from ADP and Pi, 7300 calories are required therefore the amount of energy which can be conserved by phosphorylation can be calculated as follows:
2. Anaerobic Respiration:
In this type of respiration carbon dioxide is produced but no atmospheric oxygen is used. This is therefore called anaerobic respiration, i.e., respiration without air. Anaerobic respiration is the only method of respiration in many kinds of fungi. This type of anaerobic respiration is called as the fermentation. It differs from anaeorobic respiration in that the substrate is present outside the cell in a soluble form.
Depending upon the nature of the end product, fermentation is of following types:
1. Alcoholic Fermentation:
In the year 1897 Buchner showed that the yeast extract contained an enzyme complex which can catalyse the conversion of sugar into alcohol and CO2.
This extract was named as zymase. Gay Lussac (1860) had represented the above process in the form of following equation:
The process of alcoholic fermentation can be explained as follows:
The pyruvic acid produced during glycolysis is converted into acetaldehyde and CO2 by the enzyme carboxylase which is believed to be one of the components of zymase complex. Acetaldehyde is reduced by another enzyme alcohol dehydrogenase, belonging to the same complex, into ethyl alcohol. The hydrogen atoms are donated by NADH2 produced during glycolysis. It thus regenerates NAD for smooth operation of glycolysis.
The above steps can be represented in the form of following equations:
Alcoholic fermentation may occur in almost any dilute sugar solution which is inoculated with yeast or is left exposed to air. It is of great economic importance and is applied on industrial scale for production of alcoholic beverages and in bakeries.
It is worth mentioning here that pure zymase is incapable of carrying out fermentation. It requires the presence of phosphate ions for proper activity. Secondly, starch cannot be fermented by yeast as it does not contain amylase.
2. Lactic Acid Fermentation:
Bacteria like Bacterium acidic lactici, Lactobacillus lactici (aerobic) and Bacterium lactic acidic (anaerobic) convert the milk sugar (lactose) into lactic acid. It causes souring of the milk.
This fermentation is of two types:
(i) Homofermentive:
It results into production of only one product, i.e. lactic acid molecules. It takes place in muscles during oxygen stress.
(ii) Heterofermentive:
In addition to lactic acid, ethyl alcohol and CO2 are also produced. It takes place in microbes. Thus it results in formation of more than one product.
3. Butyric Add Fermentation:
It takes place in butter and produces butyric acid which causes its rancidity.
4. Acetic Acid Fermentation:
It is brought about by bacteria like Acetobacter aceti and A. xylinum which oxidise ethyl alcohol into acetic acid. This type of fermentation is commercially used in the production of vinegar.