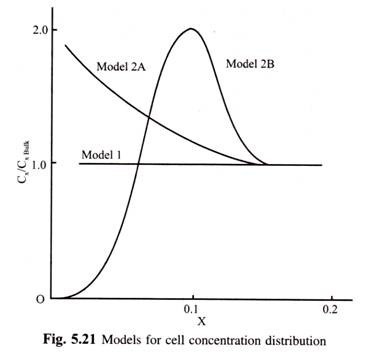
List of twenty major diseases of plants:- 1. Brown Spot Disease of Rice 2. Blast Disease of Rice 3. Green Smut or False Smut of Paddy 4. Loose Smut of Wheat 5. Black Stem Rust of Wheat 6. Late Blight Disease of Potato 7. Stem Rot of Jute 8. Ergot Disease of Rye 9. Grey Blight of Tea 10. Wilt of Pigeon Pea 11. White Rust of Crucifers 12. Tikka Disease of Groundnut and Others.
1. Brown Spot Disease of Rice:
Brown spot disease of rice is also known as helminthosporiosis, sesame spot, seedling and leaf blight. The disease is almost worldwide in distribution, but occurs severely in South-East Asia, Philippines, Japan etc. The disease is seed borne and due to non-selective export to other countries, it becomes distributed throughout the world.
In India, Sundarraman first reported the disease in 1922. Now this disease is commonly available in all rice growing areas, especially in heavy monsoon areas of West Bengal, Assam, Tamil Nadu, U.P. (eastern region) and a few regions of Kerala; mostly at the seedling stage.
The disease causes damages of different degree in different countries during epiphytotics (usually occur widely, but periodically, in a destructive form). The outbreak of the disease in Bengal was in 1942, caused up to 90% loss in yield; and thus caused Bengal famine in 1943. It was so severe that about five million (5,000,000) people died of starvation.
The loss in reduction of grain weight ranges from 4.6-29%, Later Padmanabhan (1973) discussed in detail about the famine in his article “Great Bengal Famine”.
Importance:
The disease causes great economic loss.
Three phases of damage resulting from the disease are:
1. Low rate of germination of infected seeds.
2. Leaf infection causes the reduction of photo- synthetic area, resulting in the reduction of yield.
3. Due to infection and general weakening of the plant, poor setting of seeds takes place.
Host Oryza sativa L. (Family—Gramineae i.e., Poaceae).
Symptoms:
The pathogens usually attack all parts of the rice plant at all stages of development, excepting roots. The symptoms occur on the coleoptile, leaf blade, leaf sheath, and also on the inflorescence.
1. On Coleoptile:
Symptoms on the coleoptile appear as spots (Fig. 5.6A). The spots are brown, small, pinhead to oval in shape. Rarely the spot takes the form of long streaks. In severe cases, the coleoptile becomes blighted and causes death of the seedling.
2. On Leaf Blade:
Symptoms on leaf blade appear as discrete, dark brown, elliptical to eye-shaped spots (Fig. 5.6B). A fully developed spot has a greyish brown centre surrounded by reddish brown margin; which is further surrounded by yellowish halo in early stage of development. The mature spot becomes 4-6 mm in size. In severe cases, the spots coalesce and the leaf gradually becomes dried.
3. On Leaf Sheath:
Symptom on leaf sheath is very similar to the symptoms present on leaf blade.
4. On Inflorescence:
If the inflorescence becomes infected at an early stage (Fig. 5.6C), it does not develop any grain. Depending on the stages of seed development, the degree of damage varies causing complete sterility or partial filling up of the grain. Dark brown spots are seen on the glumes (Fig. 5.6D). The endosperm becomes brown and reduces the quality of the grain.
Collateral Host:
The pathogen can survive on some grasses like Leersia hexandra, Echinochloa colonum etc.; out of season, these plants act as collateral host.
Causal Organism:
The causal organism i.e., pathogen is Drechslera oryzae (Breda, de Hann) Subramanian and Jain, (earlier called as Helminthosporium oryzae Breda de Haan) which is the asexual state of the pathogen. The sexual state is Cochliobolus miyabeanus (Ito and Kuribay) Drechsler ex Dastur.
The fungus consists of septate, prostrate and much-branched hyphae. The hyphae grow both inter- and intracellularly. These become short segmented when they grow in the host.
The conidiophores arise as lateral branches from prostrate hyphae (Fig. 5.7A). They are erect, stout, short, unbranched except at the base, where they form branches. Towards the apical region, they bear conidia at regular intervals in the grooves which become visible clearly as scar after detachment of conidia.
They are dark brown to light brown at the base and to some extent paler at the apical region. The size of conidiophores may be up to 680 µm (but in India, it is not more than 175 µm), with width ranges from 5-7 µm.
The conidia are cylindrical to ellipsoid, straight or slightly curved and slightly tapering towards the rounded end (Fig. 5.7B), smooth- walled, light brown in colour (5-10 celled) and vary greatly in size from 10-19 mµ x 45-105 mµ. The wall of the apical region is moderately thin and germination takes place by both the ends (bipolar) (Fig. 5.7C).
The perfect stage is Cochliobolus miyabeanus, obtained in culture Oapan) and disease stubbles (Mexico). They develop the perithecial fruit body (Fig. 5.7D). The perithecia are black, globose, pseudoparenchymatous, with a beak having ostiole.
Asci are long, fusiform, cylindrical, slightly curved (Fig. 5.7E) and measure 21-36 µm x 145-235 µm. Asci contain mostly 4-6 filiform ascospores present in the form of a close helix. Ascospores are hyaline or pale olive-green, 6-15 septate, 250-469 µm x 6-9 µm in size.
Disease Cycle (Fig. 5.8):
Brown spot of rice is a localised disease i.e., the pathogen is restricted to the infected zone and cannot pass long distance through the inner tissue. The built up inoculum from the infected region disseminates further and causes infection.
During dormant phase, the pathogen is prevalent on seed (both externally and internally) and also on infected plant debris.
1. From Seed:
During germination of infected seeds, the coleoptile gets infected and then gradually it affects the seedling, showing blight symptom.
The infected regions of the seedling i.e., coleoptile, leaves, etc., then produce a large number of conidia. These conidia, after dissemination by wind, fall on the different regions of the same seedling or on different seedlings and cause infection. This is the secondary cycle, which repeats several times in the growing season and enhances progress of the disease.
Towards the end of the season, the inoculum from the infected leaf may go to the inflorescence and develops infected seeds; where the pathogen may present as mycelium (inside the seed) and/or conidia (on seed).
2. From Plant Debris:
Previous year’s infected plant debris also act as source of primary inoculum.
Thus, through infected seeds and infected plant debris, disease may appear in the next season and repeat the cycle.
The disease may also progress from year to year through collateral hosts, like Leersia hexandra, Echinochloa colonum and Setaria sp.
Predisposing Factors:
1. A relative humidity of 92.5% or above favours conidial production.
2. Cloudy weather and higher day temperature (28-30°C) during the latter part of the growing season (Sept.-Oct.) are favourable for the rapid spread and development of the disease.
3. Optimum temperature for germination of conidia is between 25-30°C, minimum at 2°C and humidity of about 92% or more is the best for spore germination.
4. The best development of spots occurs in shade and high humidity. Sunlight retards the growth of spots.
Disease Management:
The disease can be controlled or reduced by the following procedures:
A. Cultural Methods:
The following cultural methods are useful in controlling the disease:
1. Eradication of Collateral Hosts:
Collateral hosts like Leersia hexandra, Echinochloa colonum and Setaria sp. should be eradicated, where the pathogen can survive out of season.
2. Sanitation:
Cleaning and burning of the previous year’s infected plant debris are effective to reduce the source of primary inoculum.
3. Irrigation Water:
During irrigation, the water should not pass from infested field to the field without disease.
4. Leaching of Metals:
Leaching down of metallic elements like Fe, Mn and K from soil during rain should be checked, otherwise the plants become more susceptible to the disease.
5. Application of Metals:
Addition of metals like K in the field during growth period, reduce the severity of the disease.
6. Spacing:
Proper spacing during transplanting also reduces the disease incidence.
7. Nitrogen Fertiliser:
Optimum nitrogen fertiliser (Urea) application, transplanting at optimum dates proved to be helpful for the reduction of disease.
B. Physical Control:
1. Sunlight:
Soaking the seeds for 24hrs in water and then drying (52-54°C) in bright sunlight reduces the source of primary inoculum by killing the activated mycelium.
2. Hot Water:
Hot water treatment at 55°C for 10 minutes gives better control by reducing the primary inoculum.
3. Cold Water:
According to Central Rice Research Institute (C.R.R.I.), Cuttack, some resistance can develop inside the seedling if the seeds are allowed to germinate in cold water.
C. Chemical Control:
1. Seed Treatment:
(a) Dry Dressing:
Dry dressing of the infected seeds with Ceresan or Agrosan GN. at 2-2.5% reduces the disease.
(b) Wet Treatment:
In japan, Seed treatment with Silver nitrate, Copper sulphate, Mercuric chloride, Phenol, Formaldehyde and Calcium hypochlorite have been found very effective in controlling the disease.
2. Foliage Treatment:
Spraying or dusting of fungicide, two to three times at regular intervals, gives effective control of the disease. Dithane Z-78 (0.2%), Dithane M-45 (0.3%), Hinosan (0.1%), Blitox (0.3%), Benlate (0.2%) and Bordeaux mixtute (5:5: 50) are used to control the disease.
D. Disease Tolerant Varieties:
It is better to cultivate disease tolerant varieties than the others, which are less costly. Singh and Sharma (1975) observed that varieties like IR-24 and Padma are comparatively resistant and can be grown in the epiphytotic areas.
A number of disease tolerant varieties have been developed in India, particularly in C.R.R.I. (Central Rice Research Institute), Cuttack, Orissa. These are CH 13, CH 20, CH 45, T 141, T 2114, IET 13238, CR 84-30, JB 83 etc.
2. Blast Disease of Rice:
The blast or ‘rotten neck’ is a most destructive disease reported from almost 80 rice growing country of the world. The crop suffers heavy losses in humid areas with high rain fall. The disease was recognised as a major problem of rice production from long back in Japan, USA and also in many other countries.
In India the disease is present in most destructive form in Jammu and Kashmir, Uttar Pradesh, Bihar, Orissa, Andhra Pradesh and Tamil Nadu. The disease is also available in other parts with less frequency.
Host:
Oryza sativa L. (Family – Gramineae i.e., Poaceae).
Symptoms:
The symptoms of the disease occur on leaf, leaf sheath, columns, nodes (column joints), panicle, branch bases and also on grains.
On Leaf:
The lesions appear as small greyish or bluish, water soaked areas of 1-3 mm in diameter (Fig. 5.9A, B). During humid weather the lesions enlarge very rapidly and become 1-15 cm long and approx. 0.5 cm broad. The mature spots are spindle-shaped showing brown or reddish brown margin surrounding a grey or whitish centre and the entire spot is surrounded by yellowish halo.
In humid weather, spots on susceptible varieties enlarge very quickly and kill the entire leaf within a few days showing burnt appearance in the field, but remain as pinhead sized brown spots in resistant varieties. In moderate resistant varieties, lesions appear as small, round or elliptical in shape surrounded by brown margin and are a few mm in length.
Leaf Sheath:
More or less similar spots are observed on leaf sheath as found on leaf.
Culms:
Brown to black spots are formed on the culms.
Rachis:
Rachis of the maturing inforescence shows brown to black spots or rings.
Ears:
Ears also show similar small spots.
If the infection occurs before grain formation, the grains are not formed and the panicles hang down. However, due to damage by necrosis of the neck tissue, the ear tends to break and fall off. Maximum damage takes place at this stage.
Causal Organism:
The causal organism i.e., pathogen of the blast disease of rice is Pyricularia grisea (Cooke) Sacc., named earlier as Trichothecium griseum, Pyricularia oryzae Cav. or Dactylaria oryzae. The perfect stage of the pathogen is Magnaporthe grisea (Herbert) Yaegashi and Uddagawa, an ascomycetous fungus.
The mycelium is septate, uni- or multinucleate, branched and hyaline to olivaceous in colour.
The conidiophores develop singly or in fascicles through the stomata (Fig. 5.10). They are simple or rarely branched, 2-4 septate, with swollen base and show truncated denticles along its length (in mature stage).
Conidia are pyriform (pear-shaped) to obclavate in shape with rounded base and narrowed tip, 2-septate, rarely 1-3 septate; hyaline or pale olive, 14-40 µm x 6-1 3 µm, with a distinctly protruding basal hylum (Fig. 5.10).
A typical lesion on leaf can develop 4,000 to 6,000 conidia/night for more than two weeks.
During germination, appresoria are developed at the tip of germ tube, either on host surface, on glass slide or on cellophane paper. They are globose, oblong or ovoid and measure 5-15 µm in diameter.
The fungus often produces chlamydospores of about 5-12 µm in diameter.
Pathogen can develop perithecia in culture (Fig. 5.11 A, B). It is named as Magnaporthe grisea, and also called as Ceratosphaeria grisea and Phragmoporthe grisea. Perithecia are dark brown to black, glabrous, elongated, with spherical to subspherical base, mostly 100-180 µm in diameter with a long neck 500-1,200 µm in length.
The neck is cylindrical of more than half of its length i.e., up to 1,100 µm. Asci (Fig. 5.11C) are cylindrical to clavate, hyaline, uni- tunicate (ascus has single wall), with short stipe measuring 60-80 µm x 10-12 µm. It contains 8 ascospores.
The asci are intermingled with paraphyses. Ascospores (Fig. 5.11D) are hyaline, biseriate, fusiform, curved with rounded ends, 1-4 septate (generally 3 septate), slightly constricted at the septa measuring 18-23 µm x 5-7 µm. Germination is bipolar (Fig. 5.11 E).
Collateral Hosts:
Saccharum officinarum, Setaria intermedia, Echinochloa crus-galii, Stenotapharum secondatum, Digitaria sanguinalis and Eremochloa ophiuroides; Arundo donax, Brachiaria mutica, Dinebra retroflexa, Digitaria marginata, Panicum repens.
Disease Cycle (Fig. 5.12):
The pathogen can perpetuate in grain both externally and internally, on straw piles and also on host other than rice. The inocula, i.e., the conidia, developed from all or any of the above sources can infect the leaf of the rice plant. The infected regions develop crops of conidia; those are disseminated and infect the different regions of the same plant and /or the other rice plants.
Towards the end of the season, the pathogen may perennate as conidia on seed and also on straw piles. Next season, perennating conidia may serve as a source of primary inoculum. Likewise, in some areas they develop perithecia containing asci and ascospores. The perithecia remain with the plant debris or straw piles. Next season, the ascospores may cause infection.
Disease Management:
The disease can be controlled or reduced by the following methods:
A. Cultural Methods:
The following practices are used to reduce the disease incidence:
1. Early Planting:
Early planting shows less prevalence of disease than late planting.
2. Sanitation:
Sanitation i.e., cleaning of the plant debris from the field reduces the source of primary inoculum, thereby reduces the disease incidence.
3. Destruction of Collateral Hosts:
Destruction of collateral hosts like Panicum repens, Leersia hexandra etc., reduces the disease incidence.
B. Chemical Methods:
Chemical of different groups are used in different ways to control the disease.
These are:
1. Seed Treatment:
The pathogen is seed borne, thereby seed treatment is essential to reduce the disease.
The seeds are treated with different chemicals to reduce the disease incidence:
i. Seed treatment with Agrosan GN (organomercurial) is effective to eliminate the seed borne inoculum present externally.
ii. Copper sulphate (20 ppm) mixed with Aureofungin (20 ppm) is effective to control seed borne inoculum.
iii. Kapoor and Singh (1982) reported that seed treatment with Benomyl (1 : 400 w/w) gives protection to the seedling by inhibiting spore germination and appresorium formation of the pathogen.
iv. Fungorene (8g / kg seed) is found to be highly effective in controlling the seed borne inoculum.
2. Foliar Spray:
Cu-fungicides are not effective during severe appearance of the disease. It causes phototoxicity, thereby reduces yield. Copper fungicide mixed with mercury compound and Phenyle mercuric acetate is highly effective as foliar spray.
C. Disease Tolerant Varieties:
The following cultivars are recommended as disease tolerant varieties : A-67, A-90 and A-200 (in Maharastra); T-141 and T-603 (in Orissa); Co-4 (in Tamil Nadu); Kamala and Kukulu (in Bihar); Jaya and Pankaj (in Madhya Pradesh), etc.
3. Green Smut or False Smut of Paddy:
The smut pathogens belong to the order Ustilaginales, but green smut pathogen of paddy does not belong to this order. Initially it was placed under the subdivision Deuteromycotina, but later it has been placed under the subdivision Ascomycotina. So, it is not a smut in true sense, thus called false smut.
Cooke (1818) was the first to report the disease from Trinnevally in South India. Since then, disease gradually proceed in different regions of India and also in other countries, like America, Burma (Myanmar), Ceylon, Philippines etc.
Host:
Oryza sativa L.
Symptoms:
The symptom is confined to the grains of ears. Due to the development of fructification of the pathogen, the grains are partially or completely covered by velvety green masses or balls (Fig. 5.13). The kernels enlarge several times than the normal one. Their colour is orange-yellow on the periphery which encircles central white region.
The surface of the ball cracks with maturity. The glumes remain unchanged and are found sticking closely to the centre of the mass which burst out above. The green ball consists of lightly interwoven mycelia along with the glumes and neighbouring host tissue.
At different stages of development the ball shows 3 layers:
1. Innermost Layer:
It is yellowish in colour, with radiating mycelium and spores are in the developing stage.
2. Middle Layer:
It is orange in colour and composed of mycelium and spores.
3. Outermost Layer:
It is green in colour, consists of mature spores.
Causal Organism:
The pathogen Ustilaginoidea virens (Cke.) Takahashi is a member under the Sub-division. Deuteromycotina; Order: Moniliales; Family: Dematiaceae. Later, with the development of knowledge on sexual reproduction, its perfect stage was placed under the Sub-division. Ascomycotina; Order: Sphaeriales; Family: Clavicepitaceae and named as Claviceps oryza- sativa Hashioka.
The green ball on the grain consists of compact septate mycelia. The lateral or rarely terminal hyphae produce chlamydospores on short sterigma. The young chlamydospores are almost round and smooth, but the mature one becomes rough, olive green colour with granular coating, measuring 3-5 µm x 4-6 µm (Fig. 5.14B).
On germination, they develop pear-shaped secondary conidia on branched and septate germ tubes (Fig. 5.14C).
Some of the green balls develop into four sclerotia in the centre. The sclerotia overwinter in field and, with the onset of favourable condition i.e., in the next season, they develop stalked stroma (Fig. 5.14A). The stalk of the stromata swells at the tip and becomes more or less globose structure bearing perithecia.
Each perithecium is flask-shaped and contains about 300 asci. The asci are cylindrical having a hemispherical apical appendage measuring 180-220 µm x 4 µm. Each ascus contains 8 ascospores. Ascospores are filiform, unicellular, hyaline, measuring 120-180 µm x 0.5-1 µm.
Disease Cycle:
The incidence of this fungus is reported in wild rice and also in many grasses. Thus, it is presumed that the primary inoculum comes from the collateral host by wind dissemination. Ascospores produced from the sclerotia serves as primary inoculum and causes primary infection.
Chlamydospores are air borne, play an important role in the secondary infection and their availability is highly significant at the time of flowering of rice plant. Infection during early flowering causes destruction of the ovary, but if it is late, grain filling takes place.
The mycelium invades the endosperm and develops a mass of spores. The disease is not seed borne, thus no success has been achieved to develop infection by artificial floral inoculation.
Disease Management:
With limited knowledge about the life cycle of the pathogen, selection of any one or more control measures becomes impossible. Destruction of collateral hosts, clean cultivation, crop rotation and steeping the seeds in brine solution are suggested to control the disease.
4. Loose Smut of Wheat:
Loose smut of wheat is a destructive disease. The disease was recognised and confirmed very early than the other wheat diseases. Maddox (1895) was the first to show the nature of the disease. Due to the characteristic black dusty appearance of diseased inflorescence, it is also called as smut, black smut, black head, blasted head and snuffy ear.
The disease is available throughout the world and is more abundant in humid and semi- humid regions, cause great loss in yield. In India the loss is much more in cooler and moist northern region than the south.
Mishra and Singh (1969) reported 3-6% loss in some newly developed high yielding varieties of wheat. Later, Gothwal et at., (1972) observed that smutted plant produce 18.7% reduction in height of plants, 22.9% less tillers, 27.5% shorter peduncles and 15.7% reduction in yield.
Earlier, Luthra (1953) estimated an annual loss of fifty million rupees only in Punjab.
Host:
Triticum aestivum L.
Symptoms:
The symptom is visible only when the plants develop ears. The ears of the affected plants emerge out of the flag leaf earlier than the healthy ones. In infected plants, generally all the ears and all the spikelets and kernels of each ear are smutted. The spikelets of the affected ear get deformed and filled with dry, black, powdery mass of spores, thus no grains are formed (Fig. 5.15B).
Initially the spores are covered by a delicate greyish membrane which soon burst and the spores get free. The spores are blown off by wind leaving only the naked rachis. Rarely some of the tillers developed from infected seed may escape from the disease. The number and length of the tiller is reduced in infected plant.
The growth and general appearance of the plant is not affected. The seeds become infected without showing any change in the external morphology.
Causal Organism:
The pathogen is Ustilago segetum (Pers.) Roussel var. tritici (earlier was known as Ustilago tritici (Pers.) Rostr.).
The black powdery mass of spores, the chlamydospores, are olive-green, spherical to oval in shape, minutely echinulate walls and 5-9 µm in diameter (Fig. 5.16A). The chlamydospore germinates to develop a promycelium of four uni-nucleate cells (basidium) and each cell later develops into germ tube or infection thread (Fig. 5.16B-D).
No sporidia are developed from uninucleate cells of promycelium. The spores remain viable for 5-6 months. The fungus is unable to survive saprophytically in the soil.
Disease Cycle (Fig. 5.17):
The mass of chlamydospores from the infected ear are disseminated by wind or any other agencies to the healthy ear of adjacent plants at the flowering stage. The spores lodge between the glumes and reach the hairy stigmas. The spores germinate on the stigmatic fluid and develop promycelia. The promycelia becomes septate and form four uninucleate cells.
The dikaryophase is established by fusion of germ tubes (infection threads) derived from the individual uninucleate cells of the promycelium and ultimately infection hyphae are developed (Fig. 5.16E). Earlier it was thought that infection hyphae penetrate the stigma of healthy flowers, but later it has been shown that normally the entry takes place through the young tissue at the base of the ovary.
After that, the infection hyphae grow intercellularly and ultimately reach the ovule. The infection hyphae then pass into the space between nucellus and the endosperm and reach the bottom of the endosperm to reach the scutellum and the embryo. The growth of infection hyphae is intercellular and haustoria are not formed. The host cells are not affected even at a minimum level.
Along with the above process of fungal growth; pollination, fertilisation and embryo formation take place in the ears. The fungal hyphae continue to grow along with the development of seeds. The fungal hyphae become thick walled and remain dormant in the seed until the next growing season.
Profuse mycelium is present in the scutellum of the mature infected seed. Healthy and infected grains cannot be morphologically distinguished. After sowing, the infected seeds will develop seedlings and simultaneously dormant mycelium becomes active and keeps place behind the growing apex.
There is a great accumulation of hyphae at each ear which ultimately replace the spikelets with masses of black spores. During dry weather, almost all the spores are blown off, leaving the rachis as a bare stalk. After dispersal, the spores again infect the healthy host and continue the cycle.
Disease Management:
The disease can be controlled or reduced by the following methods:
A. Cultural Methods:
1. Disease-free seeds. Sowing of disease free seeds will prevent the disease incidence.
B. Physical Methods:
1. Hot Water Treatment:
According to Jensen (1908), the seeds are soaked in water at 26-30°C for 4-5 hours which activate the dormant mycelium to germinate.
The soaked seeds are then quickly transferred to hot water (54°C) for 10 minutes to kill the internal mycelium. According to Gera et al., (1963), the internal fungus could be completely eliminated by soaking the seeds in water at 25°C for 41-48 hours or at 33°C for 23-29 hours.
2. Solar Energy Treatment:
According to Luthra and Sattar (1934), seeds soaked in water for four hours in the forenoon, followed by drying for four hours in the sun on a bright summer day (at 42-44°c) cause the complete eradication of the pathogen. The procedure is very simple and economical.
3. Water Soaking Treatment:
According to Tyner (1953), loose smut of spring wheat can be effectively controlled by soaking seeds in water at room temperature for 56-64 hours. The death of the mycelium takes place due to accumulation of two quinine compounds, 6- hydroxy quinine and 2, 6-dihydroxy quinine inside the soaked seeds due to anaerobic respiration.
4. Anaerobic Treatment:
Control at significant level can be done by water soaking the seeds for 2-4 hours at 15-21 °C (60-70°F), followed by keeping in airtight container for 65-70 hours and then storing after drying them.
C. Chemical Methods:
1. Dry dressing of Seeds:
Loose smut can be controlled at highly significant level by seed dressing with systemic fungicides, like Benomyl and Carboxin at 0.2-0.25%. Bavistin SD (25% a.i.), a systemic fungicide is also highly effective in controlling the disease.
2. Wet Treatment:
Significant control can be gained by soaking the seeds in water containing Sodium hypochlorite or Ceresan.
D. Biological Method (Biocontrol):
TV-5, a strain of Trichoderma viridae is a highly effective biocontrol agent to control loose smut of wheat.
E. Disease Tolerant Varieties:
Some disease tolerant varieties have been bred and recommended by Indian Agricultural Research Institute (IARI), New Delhi; these are Kalyan 227, PV 18, C-302 and WG-307. Several other tolerant varieties like NP-710. NP-718, NP-729, NP-761, NP-791, NP-81 7, NP-823, NP-865, NP-871 and MPO-117, MPO-125 are recommended for cultivation in Punjab, Bihar, Rajasthan and U.P.
Investigation on the Presence of Loose Smut Pathogen (Ustilago Segetum Var. Tritici) in the Embryo of Wheat Seed:
Presence of Fungus Inside the Embryo can be Studied by the Procedure Mentioned below:
Take 100 wheat seeds and dip in 10% KOH solution overnight. The embryo will be separated and the rest becomes a cottony mass. Take out the embryos from the solution and wash in distilled water.
Embryo pieces are taken one by one on clean glass slide containing a drop of lactophenol and then covered with cover glass. Slightly tap the cover glass. The cells of the embryonic tissue get separated and thick brown- coloured mycelium becomes visible under compound microscope, if present.
5. Black Stem Rust of Wheat:
Black stem rust of wheat causes damage of variable degree in all the wheat-growing areas of the world. The disease has been extensively studied in Europe, USA and Australia, where it often causes severe losses in yield. In India, it is available in almost all the wheat-growing areas.
The damage from this disease is much more severe in South India than North India. In southern and peninsular India, the disease may appear in the fourth week of November, hence loss is much more here than northern region, where disease may appear in early March at the time the crop reaches the ‘dough’ stage (plant with developing grain, having milky white liquid endosperm) and thus cause less damage.
It may cause about 90% reduction in yield during severe infection showing premature ripening and shrinking of the grains.
Host:
Triticum aestivum L.
Symptoms:
On Wheat:
The symptoms appear on the stem, leaf sheaths, leaves and also on ears. The damage of stem is much more severe than the other regions.
In the early part of the season, the symptoms are very common on culms, leaf sheaths and also on leaves as elongated brown pustules, the uredosori containing huge number of uredospores. The uredosori may be quarter of an inch (6 mm) , or more in length. After maturation the-uredosori burst and expose the brown powder like uredospores.
Teleutospores develop towards the end of the season in new sorus, the teleutosorus or in the same sorus along with uredospores — the mixed sorus. The teleutosori are black in colour and expose the mass of black spores — the teleutospores. The teleutosori are more frequent on the stem and other parts, but least on the leaves.
During severe infection plants fail to develop normal ears and the grains become shrivelled with light in weight.
On Barberry:
The symptoms first appear on the upper surface of the leaflet as small circular yellowish spots of variable numbers. Later on, the spots increase in size up to 5 mm or more. The mature spots are honey-coloured in the centre, margined with red to purple colour, the pycnium.
Almost towards the opposite side (i.e., on lower side) of the leaflet, yellowish to orange coloured spots, the accia or cluster cup appear.
Causal Organism:
Puccinia graminis tritici Erikss. and Henn., is a macrocyclic (i.e., large life history cycle), polymorphic (poly = many, morphs = forms; i.e., various forms in their life cycle) and heteroecious (i.e., require two different and unrelated hosts to complete their life cycle) rust.
The different hosts are wheat and barberry.
On Wheat:
Uredosori and teleutosori develop on wheat plant and basidiospores on infected plant debris of wheat.
The uredosori contain uredospores which are one-celled, dikaryotic (n + n), brown in colour, oval in shape, having thick echinulate wall and four equatorially placed germ pores. They measure 25-30 µm x 17-20 µm.
The teleutosori contain teleutospores which are two-celled (each cell is dikaryotic i.e., n + n), black, having thick and smooth wall with two germ pores, measuring 40-50 µm x 15-20 µm. The germ pore of the apical cell is situated at the rounded apex, while in the lower cell it is at one side just below the septum. The teleutospores remain with the plant debris and undergo a long period of rest. Before germination they have to be exposed to freezing temperature.
Teleutospores, on germination, produce, a long 4-celled promycelium (the septate basidium) and each promycelial cell develops one sterigma bearing one haploid basidiospore. This is the saprophytic stage of the pathogen.
On Barberry:
Pycnia and aecia are produced on barberry plant.
Pycnia (spermatangia) are flask-shaped with an opening — the ostiole. Inner surface of pycnium is lined with elongated pycnophores, develops many small, uninucleate conidia or spermatia. Towards the pore mouth many sterile hyphae, the periphyses, are present, those remain intermingled with much longer and generally forked receptive hyphae.
Aecia are inverted cup-shaped or bell- shaped in structure enclosed by a peridium. The aeciospores are one-celled, dikaryotic (n + n), echinulate having six germ pores. They develop in chain but arrange alternately with disjunctor cell from the basal end of the aecial cup. Aeciospores can infect wheat or any other grass host.
Collateral Hosts:
Many grass hosts, like Brachypodium sylvaticum, Bromus patulus, B. coloratus, B. carinatus, B. japonicus, B. mollis, Lolium perenne, Hordeum distichon, H. murinum, Aegilops squarrosa, A. trineclis, A. ventri- cosa are infected by rust pathogen. Like wheat, they grow in the winter season and, hence, the above collateral hosts have no importance in the annual recurrence of the black stem rust in the plains.
Disease Cycle:
The pathogen (Puccinia graminis tritici) can survive on straw of wheat plant, (the primary host), for several months as teleutospores, which then transfer the disease to the barberry (the alternate host).
Teleutospore, on germination, produce the basidiospores of two opposite strains i.e., 2 of (+) type and other 2 of (-) type. Basidiospores are dispersed by wind and fall on the barberry plant and infect mainly the leaves.
Pycnia (+ and – type) are produced on the upper epidermis of the leaflet by the (+) and (-) basidiospores, respectively. After spermatisation between pycnospore and receptive hypha, and with the help of raindrop or insect, dikaryotic condition is established resulting into the formation of aecia and aeciospores on the lower surface of the leaflet.
The aeciospores developed in different aecium are of the same type. After release from the barberry leaflet with the help of wind, it falls on the surface of wheat plant and after infection it produces uredosorus containing uredospores.
Then, uredospores infect different region of the same plant or the different plants and develop next crop of urdospores in the same season. This is the secondary cycle which may repeat several times in the growing season.
At the end of the growing season i.e., during February to March, infection of uredospores takes place, but instead of developing uredosorus it develops teleutosorus containing teleutospores — and thus complete the disease cycle.
In India, the above cycle does not prevail due to different environmental conditions and the absence of suitable alternate host.
Annual Recurrence of Black Stem Rust of Wheat in India:
Puccinia graminis tritici, the pathogen of Black stem rust of wheat, complete its life cycle with the help of two different and unrelated hosts — the wheat (primary host) and barberry (secondary host).
The disease appears year after year by the completion of the life cycle and causes severe damage with great economic loss.
In India, the disease also appears every year and cause great economic loss, but the scenario- is different. The possible ways of annual recurrence are discussed here based on the work of different scientists of our country.
Role of Barberry:
The role of barberry (Berberis spp.) is discussed next:
1. Berberis does not occur in the plains of India.
2. Berberis vulgaris, the highly susceptible species, is not available in the hills.
3. Only the moderately susceptible species of Berberis are available.
4. According to Prasada (1947), no Berberis infection observed in nature has been connected with black stem rust.
The above facts rule out the possible role of Berberis in the annual recurrence of Black stem rust in India.
Role of Environment:
Effect on Teleutospore:
Teleutospores are formed at the end of March to May and their subsequent exposure to intense summer heat rules out the possibility of their survival. During the appearance of new leaf of Berberis in summer, the temperature and humidity are unfavourable for the germination of teleutospores, this rules out the possibility of natural infection of Berberis by basidiospores.
Effect on Uredospores:
In Indian plains, the wheat is sown in October to November and harvested in March to May. During the early part of the growing season the conditions are favourable for infection, still the appearance of the disease is delayed by two to three months.
The uredospores developed in the plains will not be able to survive like teleutospores due to intense summer after the end of the season. The above fact rules out the possibility of uredo- spore development in plains and their role in the annual recurrence of Black stem rust in India.
Now, the uredospores developed in the hills came in focus. Wheat that grows both in the northern and the southern hills develops uredospores. Mehta (1933, 1940) observed in details regarding the survival of the uredospores out of season during summer in the hills. He observed that the disease in the new crop appears earlier in the foothills than the plains.
With the comparative studies, he also observed that there is heavier infection in the foothills than the neighbouring plains and the period of rust appearance in the plains and the foothills in different parts of the country is also different.
Mehta (1933, 1940, 1952) concluded that the inoculum of the black rust has been introduced in the plains from the hills (1,200-2,400m/4,000- 8,000 ft) where the uredospores are able to over- summer.
Due to optimum humidity and milder summer, the uredospores over summers on disease stubbles, volunteer wheat and also on the regular wheat crops of both summer and winter in the Pulney and Nilgiri hills in the south and the sub- mountainous regioh of the Himalayas in the north.
Later observation also includes the Panchgani- Mahabaleshwar range of Western Ghats as another focus of infection. He concluded that the inoculum for the infection in plains comes from north as well as from south.
Later, Joshi et al, (1974) observed that South India gets black stem rust infection earlier than the north. Although the pathogen can survive in the north hills throughout the summer months, a number of factors seem to indicate that the inoculum of the northern hills does not play any significant role as the primary inoculum for the black stem rust infection of the northern plains.
The main reason is the temperature of the northern hills. The average minimum temperature in north India from December to February ranges from 7-13°C. The incubation period of the uredospore in this temperature is 40-50 day in January and February, thereby very little time is left for infection as the crop reaches maturity.
The southern inoculum develops earlier and its dissemination towards north is the possible principal source of inoculum for the northern wheat belts through Karnataka, M.P., and Maharastra, where the inoculum multiplies much more.
Disease Management:
The disease can be controlled or reduced by the following methods:
A. Cultural Methods:
1. Considering the annual recurrence of black rust in India, Mehta (1929) suggested the suspension of summer crop in the Nilgiri and Pulney hills and replacement of wheat and barley cultivation by oats in the hills.
2. Potassium increase the resistance of wheat to rust infection, but nitrogen has the opposite effect. Reduction of nitrogen in NPK ratio helps in reducing the rust incidence in susceptible varieties.
3. Mixed cropping of wheat with non- cereal crops (mustard) reduces the amount of inoculum for secondary infection and further spread of the disease.
B. Chemical Control:
1. Forsyth and Peturson (1958) observed an effective control with 4-5 times application of Zinc sulphate and Nabam.
2. Experiments done in U.P. by sulphur dusting at the rate of 13.6 kg/acre at an interval of 4 days during the appearance of the disease give good control. To avoid the wastage of sulphur which needs foreign exchange to import, an efficient forecasting system is required.
3. Wallen (1958) proposed to control the rust through the application of an antifungal antibiotic, the cycloheximide, at the rate of 50 ppm (182 It/acre) as a systemic fungicide.
4. Crewal and Dharm Vir (1959) observed that good control of stem rust is possible by spraying of Parazate liquid + ZnSO4, 4 times at fortnight intervals.
5. Forsyth and Peturson (1958) reported that effective control of stem rust can be obtained with 4-5 times application of ZnSO4 and Nabam.
6. Edgington and Reinberg (1966) reported that Plantavax and Vitavax are very effective as seed, soil and foliar spray treatment to control stem rust.
7. Brahma and Asir (1988) observed that Propiconazole (Tilt) at 0.1% is effective to control the stem rust by inhibiting completely the germination of uredospores.
C. Disease Tolerant Varieties:
Some varieties are very useful to prevent most of the races of stem rust pathogen. These are S 227, S 307, S 308, S 331, Sonara 63, Sonara 64, Lerma Rojo, HD 2278, WL 614 and HW 741.
6. Late Blight Disease of Potato:
The disease causes blight symptom which appears towards middle to late in the season, that’s why the name is given. Potato is a native of the North Andes in South America. Initially, late blight of potato was available in that area as an endemic disease. Gradually, by 1840-1847, it spread throughout USA and Europe.
The disease became well established in Ireland, Europe and England by 1842. Later, in 1845, it spread as epiphytic disease throughout the Europe and brought famine in Ireland, causing the death of one million (1,000,000) people and more than one million migrated to other countries.
In India, the Late blight was introduced in the Nilgiri hilts between 1870-1880 from England. Now-a-days, the disease is frequently available in the potato growing areas of India.
The loss from this disease is less in West Bengal and Bihar (5-10%), which gradually increases in Punjab (20-25%), U.P. (15-50%) and Haryana (40-50%) and causes a monetary loss of Rs. 4 crores per year. The loss is gradually reduced by the introduction and popularisation in cultivation of Kufri Chandramukhi, and some other varieties.
According to A. Basu (2002), some exotic Dutch cultivars like Cardinal and Diament show better resistance along with good yield in West Bengal.
Host:
Solanum tuberosum L.
Symptoms:
Symptoms of Late Blight of Potato are Available on Foliage and Inside the Tuber:
1. On Foliage:
Symptoms appear as hydrotic areas with indefinite margins at the apex and edges of the leaflets (Fig. 5.18A). The infected regions gradually turn brown to black due to necrosis. In early stage of disease development, chlorotic border develops around the necrotic regions.
During moist weather, the disease progresses very rapidly, causes much more decay of the infected regions and produces a characteristic bad odour. The sporangiophore and sporangia are visible as matty growth on the abaxial surface of the leaflet. The entire plant may be damaged completely within a few days.
During dry weather, the disease progresses very slowly and the affected regions curl and shrivel. The diseased areas become hard and easily break with small disturbance.
2. Inside the Tuber:
The pathogen does not go down through the stem to the tuber.
The Tuber Infection takes place in different ways:
(a) During Growing Season:
Hydrotic areas develop on the tuber surface and the regions become necrotic. If condition favours, the entire potato turns brown and becomes damaged completely before harvest.
(b) During Harvest:
During harvesting, the tuber with delicate skin may get ruptured and the tuber may find contact with the infected leaf and cause infection. Later, the symptom is visible after cutting the tuber as a wheel marked by small brown dots (Fig. 5.18B).
Causal Organism:
The causal organism, Phytophthora infestans, belongs to the subdivision Mastigomycotina under the Division Eumycota. The plant body is a coenocytic mycelium which grows both intra- and intercellularly inside the host tissue. The sporangiophore develops either singly or in groups on the young seedling and also from the lower side of the leaf of adult plant (Fig. 5.19A, B).
It is branched and develop sporangia at the apices. Initially, one sporangium develops at the apex (Fig. 5.19C) and, at maturity, the sporangiophore elongates from one side and shifts the sporangium towards the opposite side — another sporangium develops at the apex.
The sporangia are lemon-shaped and colourless with a small stalk below and thin papilla above (Fig. 5.19D). During favourable condition sporangium produces a number of biflagellate secondary zoospores (Fig. 5.19E). After swimming for some time in dewdrop or thin film of water, the zoospores encyst and then germinate by producing germ tube (Fig. 5.19F).
The germ tube penetrates directly through stomata or develop appresorium at its tip (on contact with cell wall), which then penetrates the host wall both by mechanical pressure as well as by enzymatic action. During unfavourable condition the sporangium behaves as conidium, which germinates directly by producing germ tube.
Optimum temperature for the growth of mycelium is 16-18°C, for sporulation is 21 °C, for germination of sporangia by zoospore is 12°C, while at 21 °C it germinates directly by germ tube.
Relative humidity of 100% favours the abundant production of sporangium, while below 90% RH inhibits its production.
The fungus is heterothallic. During sexual reproduction oospores are produced inside the aerial parts by the union between antheridium and Oogonium (Fig. 5.19G).
Disease Cycle (Fig. 5.20):
In the dormant phase, the fungus could perennate in the tuber as mycelium and in plant debris as oospore. During favourable condition, the perennating mycelium becomes active and develops active mycelium. The active mycelium develops branched sporangiophore bearing sporangia on it.
On the other hand, the oospore germinates by producing germ tube bearing sporangia at their tip. In both the cases, the developed sporangia disperse by wind or rain, germinate on host surface by producing zoospores and cause infection to the host tissue.
The infected host again develops sporangia on sporangiophore. The sporangia develop zoospore which cause further infection. This process repeats several times in the growing season — this is the secondary cycle.
During favourable condition, the zoospores may come down into the soil and may cause infection to the tubers. The tuber infection may also take place on contact with infected foliage during harvest.
The perennating mycelium inside the tuber remains active, if the infected tubers are kept in the storage for seed potato. In the next season, after sowing the seed, the perennating mycelium becomes active and causes further infection.
Towards the end of the season, inside the aerial parts, both the sex organs — antheridium (male) and oogonium (female) — develop and undergo sexual reproduction of amphigynous type (Fig. 5.19G).
The product of sexual reproduction is oospore, which has hard protective covering. The oospore remains dormant inside the host tissue during unfavourable season. During favourable condition in the next season they germinate and produce next crop of zoospores.
Oospore formation is very rare in USA and Canada and may not play a significant role in the survival of the fungus. But it is frequently available in Mexico and indicates the possibility of playing significant role in the annual recurrence.
But in India, the potato-growing season is followed by hot summer season in the plains, ruling out the possibility of survival of inoculum in the soil and also in the plant debries. The perennating mycelium in the tuber is killed by exposure at 40°C for 4 hours or at 30°C for 65 hours. Thus, in plains, the tubers stored in the house at room temperature becomes free from disease.
In the northern hills and also in its surrounding plains, the mycelium may remain active due to high humidity and low temperature and causes disease in more severe form. The disease also appears with more intensity, possibly due to survival of inoculum inside the seed tubers kept in cold storage.
Disease Management:
The disease can be controlled or reduced by the following procedures:
A. Cultural Methods:
The methods useful in controlling the late blight of potato are:
1. Seed tubers should not be collected from the disease field.
2. Tubers should be harvested after the maturity of the tubers (when skin of the tubers comes tough).
3. In infected field, the aerial part of the plants should be dried completely before harvest of tubers.
4. Infected plant should be cut out and collectively buried deep in the soil.
5. The previous year’s plant debris should be cleaned from the field and it must be burnt outside the field.
6. The volunteer potato plants that may harbour the infection mainly in hills should be eradicated during appearance.
7. Planting of alternate row of susceptible and resistant variety will reduce the intensity of the disease.
8. Cultivation of tuber on high ridges and covering the tuber with soil will prevent the fungus to grow out from the infected mother tuber, thus reduce the spread of the disease.
9. The nitrogen fertiliser should be applied at a reduced rate.
10. During harvesting, the potato plants should be placed on one side and tubers on other side, thereby contact between tuber and infected plant can be avoided.
B. Physical Methods:
Following methods are used to control the disease:
1. The perenating mycelium in the tuber can be killed by exposure to 30°C for 65 hours or to 40°C for 4 hours.
2. The perenating mycelium can be killed by dipping the tuber in water at 45°C for 4-5 hours or at 40°C for 24 hours.
C. Chemical Methods:
1. Dutt (1962), while working with 16 different fungicides in hilly region, found that Bordeaux mixture (5:5: 50) is the most effective giving better result than. Burgundy mixture.
2. Fungicides like Brestan (1 kg/ha), Dithane M-45 (2 kg/ha) and Difolitan 80WP (2.5 kg/ha) are used as foliar spray and found highly effective in controlling late blight disease of potato in plains of India.
3. According to Khanna and Sharma (1981), Metalaxyl (1 kg/ha), a systematic fungicide, is found highly effective in controlling the disease, thus increasing the yield about 3 times than the control.
D. Biological Methods (Biocontrol):
1. Jindal et al., (1988) reported the role of Epicoccum purpurascens, Trichoderma koningii and Stachybotrys atra in controlling the disease.
2. Roy et al. (1991) reported the role of Penicillium aurantiogriseum and Myrothecium verrucaria in late blight disease management.
3. Arora (1999) reported the role of Penicillium viridecatum, Chaetium brasilense and Trichoderma viride in controlling the late blight disease. E. Disease tolerant varieties. It is better to use tolerant varieties.
In India, Central Potato Breeding Station (Simla) has developed some tolerant varieties like Kufri Jyoti, Kufri Naveen, Kufri Badshah, Kufri Jeevan, Kufri Neela etc. for commercial cultivation. Cardinal and Diament (Dutch cultivrs) and Kufri Chandramukhi and Kufri Chipsona-1 (Indian cultivars) are the varieties showing good performance in alluvial zone of West Bengal.
7. Stem Rot of Jute:
The disease is commonly observed in Assam, West Bengal, Orissa, Bihar and eastern part of U.P. The disease is also common in the jute growing regions of Bangladesh.
Host:
Corchorus capsularis L. (Tita jute) and C. olitorius L. (Mitha jute).
Symptoms:
Plants are attacked mostly on all stages of growth. But the damage from this disease may be severe in seedling stage and also in the adult plants.
Symptoms are Visible on the Following Regions:
On Seedling:
If seedling develops from infected seed, it becomes infected at different regions, such as root, hypcotyle zone, cotyledon and also in the apex (Fig. 5.21 A). Infection at any region of the seedling causes death within few days, due to quick progress of the pathogen in the soft host tissue.
If inoculum comes from soil, it may infect and damage the collar region of seedling, thereby the seedling will show the damping off symptom i.e., toppling down of the seedling. Normally “damping off” symptom appears up to 20 day old seedling. After 20 days i.e., 21 days onwards, the seedling becomes infected at collar region, which does not show damping off symptom.
On Older Plants:
On older plants, the leaves are attacked. Symptoms are visible on the apex and also on the margin of leaves, which gradually proceed towards lower side (Fig. 5.21C). The leaves then fall down leaving the jute plants with bare branches.
With severe infection, the stems rot and the roots become shredded. On the stem, epidermis ruptures, the fibres become exposed and damage both the length and strength of fibres (Fig. 5.21 B).
On Fruit:
The infected capsules become black. The seeds become small and discoloured. Sclerotia of the pathogen are often visible on the fruit surface as black dots.
Causal Organism (Pathogen):
The pathogen is Macrophomina phaseolina (Tassi) Goid. The sclerotial stage of the pathogen is Rhizoctonia bataticola (Taub) Butler.
The vegetative body consists of profusely branched and septate mycelium (Fig. 5.22A). The sclerotia are small, black-coloured, irregular in shape (Fig. 5.22B) and vary in diameter from 40-85 pm. On germination, it develops mycelia from all side of the body. The Pycnidia are globose and contain profuse oval-shaped pycnospore (Fig. 5.22C) which develop on each pycnophore. Pycnospores measure 16-27 µm x 6-10-µm.
Disease Cycle (Fig. 5.23):
The pathogen can survive in soil and also on host (either living or dead) — so it is called facultative parasite.
The pathogen may remain as dormant both in infected seed and infected plant debris of the previous year.
If the seeds are infected, depending on the amount of inoculum, they may germinate or remain ungerminated. The ungerminated seeds will increase the amount of inoculum in the soil after sowing. The germinated seeds develop the infected seedlings, which later die and also increase the inoculum in soil.
The previous year’s infected plant debris will also increase the inoculum in the soil. Inocula from the above three sources may reach the various hosts like cotton, tobacco, potato, sesamum, mulberry etc., along with the jute plant. The inoculum may come to the jute plant from infected host other than jute, in addition to the above three sources.
Pathogen from infected root of jute plant may pass to the root of other healthy jute plant and to the stem and fruit to the same plant by upward movement. Inoculum from the host may reach the jute leaf and its stem gets the infection by passage of inoculum through the petiole.
Pycnospores developed on stem may again infect the leaf of the same or of different plants. Gradually the fruits become infected, which develop the infected seeds and complete the cycle.
Disease Management:
The disease can be controlled or reduced by the following procedures:
A. Cultural Methods:
1. Sanitation:
Cleaning of the previous year’s infected plant debris should be done to reduce the primary source of infection.
2. Drainage:
Proper drainage of water should be maintained this reduces the damping off incidence.
3. Spacing:
Proper spacing between the plants reduces the spread of the disease. More space may reduce disease, but it induces branching of jute, which will create problem during fibre extraction.
4. Disease-Free Seeds:
Sowing of disease- free seeds will reduce the disease incidence.
5. Role of Macronutrients:
Swada (1916) observed that the application of potash was effective in checking the disease incidence in Formosa. Ghosh and Basak (1965) found that application of nitrogen alone incites the disease incidence and it will increase to a maximum level, at 80 kg/ha.
6. Role of Micronutrient:
Thakurji et al. (1976) reported the positive reduction of disease incidence due to the application of-micronutrients alone or in combination with phosphorus, potash or nitrogen.
B. Chemical Methods:
The disease is both externally and internally seed-borne. So, the seed treatment is very effective to reduce the disease incidence. Seed treatment with Bavistin (2 kg/ha), a systemic fungicide, is very effective to control the disease.
C. Disease Tolerant Varieties:
No variety is available in the existing germplasms, which shows absolute tolerance. But Jute Research Station, Kendrapara, Orissa, reported the following varieties as field tolerant against the disease: C-58-9433, JRC-9823, Bangkak, Halmhera and Patchy albino of capsularis jute and JRO-3331 of olitorius jute.
8. Ergot Disease of Rye:
The disease occurs throughout the world. In addition to rye the pathogen also grows on bajra, wheat, grasses, barley and oats. About 5-10% of the grains of infected inflorescence become damaged by the pathogen. On infected inflorescence, the ovary becomes replaced by the elongated sclerotium.
The sclerotia have great economic importance both from harmful and useful sides. Though the disease does not reduce much in the yield, it causes various damages to human beings and also to different lives-tocks.
A. Harmful Activities:
1. In human being: Consumption of sclerotium powder along with rye-flour, results in gangrenous loss of extremities or convulsions, paralysis, hallucination and, finally, death.
2. In livestock: The livestock are sometimes badly poisoned by eating the sclerotia along with the Red Hop and other kinds of hay. They may show loss of teeth, hoofs, hair, tail and horns. They also show miscarriage and nervous symptoms.
3. Production of L.S.D. (d-lysergic acid diethylamide): The well-known hallucinogenic drug (L.S.D.) is extracted from the sclerotia of the pathogen.
B. Useful Activities:
1. In Human Being
Out of several alkaloids accumulated in sclerotium, Ergometrine and its semisynthetic analogue methyl ergometrine and methyl ergometrine maleafe have prominent uterine action, they control haemorrhage of mother during childbirth, having side-effect with increase in blood pressure and decreased milk secretion.
Host:
Secale cereale
Symptoms:
Symptoms of the disease appear in two stages:
1. Early Stage of Flowering:
Yellow sticky fluid, secreted from the infected ovarian wall called “honey due” as it is sweet in taste. The fluid contains innumerable number of minute conidia of the pathogen. This conidial stage is also called “sphacelial stage” as the pathogen was initially named as Sphacelia segetum.
2. Later Stage:
With the progress of infection the ovarian tissue becomes completely destroyed. Thus the ovary becomes completely replaced by the hard horny structure, made up of fungal mycelia (Fig. 5.24).
The sclerotia are black, elongated and much longer than the size of the normal grain. They protrude out from the glumes. The number of sclerotia varies from 1-many on an inflorescence.
Causal Organism:
The name of causal organism i.e., pathogen, is Claviceps purpurea (Fr.) Tul. In the “sphacelial stage” the pathogen produces innumerable number of conidia. The conidia are minute, oval and uninucleate; those develop singly on the conidiophores present on the outer wall of the infected ovary (Fig. 5.25A).
At the later stage, the infected ovarian wall becomes converted into a hard horny structure, the sclerotium (Fig. 4.6A).
The sclerotium germinates in the next season and develops 1-60 flesh coloured, 0.5-2.5 cm stromatic stalk with a spherical head (spheridium) of pink or orange colour (Fig. 4.6C). At the inner peripheral tissue of the spheridium, there develops numerous flask-shaped perithecia that are arranged side by side (Fig. 4.8D).
Each perithecium contains a number of clavate or cylindrical asci intermingled with paraphyses. Each ascus contains 8, long, filiform, slender, hyaline and continuous or septate, ascospores (Fig. 5.25B, C) measuring 50- 76 µm x 0.6-0.7 µm.
Disease Cycle (Fig. 5.26):
The fungus perennates as sclerotium in the soil or they come back to the soil along with the grains during sowing. They can tolerate very low temperature. The sclerotia remain dormant for a few weeks to months and then germination takes place. The sclerotia do not remain viable for more than one year.
Entire or even the broken sclerotium is able to germinate and can develop stalked spheridium. The number of sclerotium varies from 1 -60. Perithecia are developed inside the spheridium. The perithecium contains many asci and each ascus contains filiform ascospores (8), which come out through the ostiole.
The ascospores are dispersed by wind or insect and infect the stigma. After germination, the ascospore develops mycelium. The mycelia go inside the ovary and form the sphacelial stage, where huge number of conidia are produced. The conidia are dispersed by wind and cause further infection. This is the secondary cycle. This cycle repeats several times in the growing season.
Disease Management:
The disease tan be controlled or reduced by the following methods:
A. Cultural Methods:
1. Sowing of Ergot-Free Seeds:
The seed lot can be made ergot-free by soaking the seeds in 32-37% potassium chloride or in 20-32% sodium chloride solution in a large tub. The seeds will sink at the bottom and the sclerotia will float on the solution and can be separated easily. Immediately the seed lot should be washed in water and then air dried.
2. Sanitation:
Cleaning the susceptible wild cereal hosts from the surrounding field, reduces the source of secondary inoculum.
3. Crop Rotation:
Rotation of rye with legumes reduces the disease at a very significant level, because the sclerotium can survive in the soil for one year only.
4. Deep Ploughing:
Deep ploughing buried the sclerotia, prevents their germination.
9. Grey Blight of Tea:
Like other diseases, Grey blight is an important disease of tea leaves. This disease is available in moderate to severe form in Africa and Asia. It is also available in South America and Australia. In India, the disease is also common in many tea gardens of Darjeeling, Dooars, Tarai, Brahmaputra valley and Nilgiri hills.
Host:
Camelia sinensis (L) O. Ktz.
Symptoms:
Symptoms appear as small brown leaf spots (Fig. 5.27A). The spots gradually enlarge and become the size of 1 cm or more in diameter. The mature spots show greyish centre with light to dark brown margin.
The spots are mostly irregular and some of them coalesce to form grey patches of irregular outline, which blight the infected leaf, hence the name grey blight. Concentric rings of dark and light coloured lines are visible on upper surface due to the development of dark coloured acervuli.
Although the leaves are infected at any stage of development, it is much more in older leaves.
Causal Organism:
Pestalotiopsis theae Swada (formerly known as Pestalotia theae). The vegetative body is composed of septate, much- branched hyaline hyphae. The hyphae grow both intra- and intercellularly inside the host tissue.
It develops acervulus in the darker zone of concentric rings of infected region and is available on both the surfaces. The acervuli are globose to lenticular in shape, which rupture the epidermis with wide and angular opening.
The acervulus contains huge number of conidia (Fig. 5.27B). The conidia are straight, fusiform in shape and 5-celled. Out of 5 cells, the median 3 cells are darker in colour (Fig. 5.27C). The condia have long hyaline apical appendages with swollen tip. The appendages are usually 3, rarely 2 or 4 in number. The conidia measure 22-23 µm x 6-7 µm and the coloured region being 18-21 µm long.
Disease Management:
1. During winter, pruning should be done carefully and the infected leaves should be cleaned out from the field and must be burnt in the open. After pruning, one-time spraying of Bordeaux mixture gives good protection from the disease.
2. Two applications of Bordeaux mixture, 1st one in winter and 2nd one in April, has been suggested, where pruning has not been done.
List of other Important Diseases of Tea:
1. Red rust. Cephaleuros parasiticus Karst. and C. mycoidea Karst., both are algal parasite.
2. Copper blight. Guignardia camelliae (Cooke) Butler.
3. Thread blight. Corticium koleroga.
4. Brown blight. Glomerella cingulata (Stonem) S. and V.S. (Syn. Colletotrichum camelliae Mass).
5. Canker. Nectria cinnabarina (Tode) fr.
6. Stump rot. Ustulina deusta.
7. Root rot. Rosellinia arcuata Petch and Ustulina zonata (Lev.) Sacc.
8. Brown rot. Hymenochaete noxia Berk.
9. Red root. Sphaerostilbe repens Berk, and Br.
10. Wilt of Pigeon Pea:
The disease was first reported by E. J. Butler (1906) in India. It causes severe damage, mostly in the dry regions of India, wherever the crop is grown — Uttar Pradesh, Bihar, Tamil Nadu, Maharashtra etc.
According to the survey report of ICRISAT (Andhra Pradesh in India), during 1975-1980, the disease appeared with maximum intensity in Maharashtra (22.6%). Other places like Uttar Pradesh and Bihar suffer from about 5-10% disease incidence. The disease incidence is very high during flowering and fruit development period.
Host:
Cajanus cajan (L.) Millsp. (Family- Leguminosae i.e., Fabaceae).
Symptoms:
The disease appears as wilting in early stages of plant growth (4-6 weeks old), characterised by sudden or commonly by general yellowing and withering of leaves following drying of the entire plant. The disease becomes severe during flowering and pod development.
It is a systemic disease, appears after the lateral roots get infected by the hyphae develop either from conidia, chlamydospores or ascospores. The infection progresses through the xylem vessel where the mycelia collectively plug the vessel and produce conidia and chlamydospores.
Later on, the woody tissue becomes brown to black in colour. Partial wilting is also visible when one side of the root system and stem becomes affected by the pathogen.
Causal Organism:
The-disease is caused by the pathogen Fusarium udum Butler. The vegetative body is mycelium. The pathogen is a soil- borne facultative parasite. It enters through roots and becomes systemic in plant. The pathogen grows both inter- and intracellularly. The pathogen completely of partially plugs the vessels and interferes the flow of water and causes wilting of the leaves.
The mycelium produces 3 types of spores, both in culture medium and in the host tissue:
These are:
1. Macroconidia:
The macroconidia are linear, thin-walled, curved with pointed ends, notched at the base, 4-5 celled measuring 15-50 µm x 3-5 µm (Fig. 5.29B).
2. Microconidia:
The microconidia are small, curved or elliptical, 1-3 celled and measured 5-15 µm x 2-4 µm (Fig. 5.29A).
3. Chlamydospores:
The chlamydospores are spherical to oval, thick-walled structures; develop either singly or in chain of 2-3 (Fig. 5.29C). They develop either terminally or intercalary. These spores can survive for many years.
The perfect stage of the pathogen, named Cibberella indica, was reported by Rai and Upadhyay (1982) found to grow commonly in aggregated form on the exposed roots and collar region of the plant. They grow superficially on the exposed host tissue. The mature perithecia are superficial, globose to subglobose, sessile, smooth-walled, dark violet in colour and measured 350-550 pm in diameter (Fig. 5.30A).
The asci are mostly sub-cylindrical, slightly broader in the middle and contain 8 ascospores (Fig. 5.30B). The ascospores are elliptical to oval, hyaline, 2-celled (Fig. 5.30C), but rarely 3-4 celled. On germination they develop germ tube which produces both macro- and microconidia (Fig. 5.30D).
Disease Cycle (Fig. 5.31):
The pathogen can survive in the soil saprophytically in absence of living host for 3-4 years. The asexual stage is more important and common in nature. The asexual spores like macro- and microconidia can survive in soil for short period, but chlamydospores can survive for long period of time.
All the spores develop mycelium on germination and infect a new host. The infection takes place through the fine lateral branches of root and grows both inter- and intracellularly. With further growth, it establishes colonisation in the xylem vessels and completely or partially blocks the water flow, thereby the plant becomes wilted.
After the death of the host plant, the pathogen passes in the soil and can survive for several years. The duration of survival of ascospores is not yet fully known. The pathogen can survive saprophytically on dead plant debris.
Besides the blockage of xylem vessel by the fungal mycelium, the blockage also takes place due to the production of toxin, fusaric acid. In addition to the above, the pathogen also produces the enzymes like cellulase, pectin methyl esterase and polygalacturonase involved in disease development.
Disease Management:
The disease can be controlled or reduced by the following procedures:
A. Cultural Methods:
The following cultural methods are used to reduce the disease incidence:
1. Crop Rotation:
It is the best way to eliminate the soil borne infection. Rotation of 4-5 years is very useful to control the disease. Crop rotation with tobacco is very much effective, possibly due to action of root exudates on pathogen.
2. Soil Management:
Deep ploughing in the summer months is very useful to eliminate the pathogen.
3. Soil Solarisation:
Soil solarisation is done by covering the soil with transparent polythene sheet of about 100pm thick for 6-8 weeks during summer months (April-May), which increases the soil temperature 6-10°C more, causes the reduction of the pathogen.
4. Mixed Cropping:
Mixed cropping with Jowar (Sorghum) or Crotalaria medicaginea is found to be very much effective in controlling the disease.
B. Chemical methods:
Application of Bavistin (2000ppm) as soil drench 10 days before inoculation is very much effective to control the disease. Fungicides, like Dithane Z-78, Zinocap and Brassicol are also effective soil drench.
C. Biological Methods:
Vasudeva et al. (1952, 54) demonstrated that Bacillus subtilis produces an antibiotic, bulbiformin; which inhibits the growth of Fusarium udum. The disease appears more after October, due to the reduction of B. subtilis population. The antibiotic production can be enhanced by soil amendment with cotton and groundnut cake.
D. Disease Tolerant Varieties:
The varieties recommended to cultivate as disease tolerant are NP (WR)-1 5, DDN-1, ICP 4769, ICP 7182, ICP 8863, ICP 9168 etc.
11. White Rust of Crucifers:
White rust of crucifer is a common disease of several cruciferous crops like mustard, cabbage, radish and turnip; found everywhere in India. The disease is also found in cruciferous weeds like Capsella bursa-pastoris and Nasturtium indicum. Saharan et al. (1984) reported that up to 54.5% loss in yield may take place due to floral infection.
Host:
Brassica campestris L., B. alba Hook & Thom, B. nigra Koch, Kaphanus sativus L., etc.
Symptoms:
All parts except the root are attacked by the pathogen. Infection is both localised and systemic. Localised infection takes place in adult plant and systemic infection in young one.
Due to localised infection, white or cream yellow coloured pustules of various shapes and sizes commonly appear on the surface of the leaves (Fig. 5.32A) and also on stem and inflorescence. Generally spots are developed on the lower surface of the leaves. In some plants, the infected leaves become thick, fleshy and inrolled. During severe infection, the growth of leaves and sometimes the entire plant become stunted.
When the young stem and inflorescences are infected, the infection becomes systemic in the tissues and stimulates hypertrophy and hyperplasia resulting into the enlargement and varied distortion of the infected organs. The inflorescence axis (rachis) becomes distorted, thus suppressed the development of flower or causes malformation and discolouration of floral parts (Fig. 5.32B, C).
Causal Organism:
White rust is caused by Albugo Candida (Lev.) Kunze, also known as Cystopus candidus Lev. It is an obligate parasite. Mycelial plant body is aseptate, grows intercellularly within the host tissue and develops globular haustoria within the adjacent cell to draw nutrition (Fig. 5.33B, C).
The mycelium grows intercellularly and forms knob-like haustoria in host cells. The hyphae from the internal mycelium collect under the epidermis. From this underlying hyphae numerous short sporangiophores are developed like a closely compact palisade layer under the epidermis. With further growth blister-like areas developed on the epidermis are called sori.
The sporangia developed on sporangiophore are arranged in basipetal succession (Fig. 5.33A). Gelatinous pad developed between the successive sporangia functions as disjunctor. With further growth pressure exerted inside the epidermis, caused the epidermis to rupture and the sporangia are exposed.
Sporangia are hyaline, more or less spherical and measure 14-16 µm x 16-20 µm. They are usually disseminated by wind. The sporangia usually germinate by zoospores or rarely by germ tube. The zoospores are reniform, biflagellate with laterally inserted flagella.
After a period of swimming, they are encysted and then germinate to form germ tube. The germ tube, on contact with stomata, penetrates the host tissue and causes infection.
At the end of growing season, oogonia and antheridia are formed from the internal mycelium in the intercellular space and by union they develop oospore (Fig. 5.33D). The oospores are globular and 40-45µm in diameter. After a long period of rest, they germinate by the formation of zoospores (Fig. 5.33E).
Disease Cycle (Fig. 5.34):
The pathogen perennates in soil as oospore either in plant debris or in soil, which serves as primary inoculum. With the onset of favourable condition, oospores germinate by zoospores and cause further infection. The sori are developed in the infected region and further develop sporangiophore, sporangium and finally zoospores, those can cause further infection.
This cycle repeats several times and spreads the disease more and more. At the end of growing season, oospores are developed inside the host tissue and remain dormant till the next season.
The infection progresses at a faster rate if temperature becomes less than 15°C and humidity becomes more than 65% in months Dec.15- Feb.15.
Disease Management:
The disease can be controlled or reduced by the following procedures:
A. Cultural Methods:
1. Sanitation. Previous year’s plant debris should be cleaned from the field and must be burnt in the field.
2. Destruction of weeds in and around the field eliminates the source of primary inoculum.
3. Crop rotation is a very good way to reduce the disease incidence.
B. Chemical Control:
1. The fungicides used to control the disease include Bordeaux mixture (0.8%), Dithane M-45 (0.2%), Daconil (0.1%) and Difolatan (0.3%). Kapoor and Sugha (1955) recommended Aliette and Ridonil to control white rust of crucifer.
12. Tikka Disease of Groundnut:
The disease, caused by Cercospora, is also known as Cercospora leaf spot. It is available in all the groundnut growing areas of India and caused about 20-50% reduction in yield. The size as well as the quality of the nuts are generally affected.
Host:
Arachis hypogea L.
Symptoms:
All the aerial parts of the plants attacked by the pathogen shows characteristic symptoms. Initially, the lower leaflets get infected showing dark spots and later on, each spot becomes surrounded by yellowish halo. Large number of spots, almost circular in outline, develops on the leaf. With severity and maturation, the spots become dark brown to almost black, particularly on the upper surface of the leaflets (Fig. 5.35).
Stems and petioles are also with spots but less in number.
With severity of disease, the spots become coalesced and defoliation of the leaf takes place. Due to defoliation, the size and quality of the nuts become greatly reduced. Infection at younger stage causes defoliation and nuts fail to develop in them. Due to this disease, the ultimate effect is the greater loss in yield.
Causal Organisms:
Cercospora arachidicola Hori (perfect stage Mycosphaerella arachidicola W. A. Jenkins) and Cercosporidium personatum (Berk and Curt) Deighton, earlier known as Cercospora personata (Berk and Curt) Ell and Eve (perfect stage Mycosphaerella berkeleyii W. A. Jenkins.)
1. Cercospora Arachidicola (Early Leaf Spot):
The mycelial plant body grows both externally and internally, without any haustorium. The mycelia by aggregation form stroma (Fig. 5.36A). The conidiophores are normally amphigenous, present only on the upper surface in younger spots.
The conidiophores are geniculate, septate or aseptate, those develop hyaline or slightly olivaceous coloured, 4-13 septate, obclavate and often curved conidia (Fig. 5.36B). Conidia measure 38-108 µm x 2-5 µm.
The perfect stage of the pathogen is Mycosphaerella arachidicola. The ascostroma is perithecial in nature, grows scattered, commonly along the lesion margin, partly embedded in the host tissue, ovate to nearly globose in shape, black, ostiolate and measure 47-84 µm x 44 -74 µm. Perithecia contain asci without paraphyses.
Asci are cylindrical club-shaped with short stipes, bitunicate, 8-spored and measure 27-37.8 µm x 7-8.4 µm. Ascospores are 2-celled, slightly curved, upper cell somewhat larger, hyaline and measure 7-15 µm x 3-4 µm.
2. Cercosporidium Personatum (Late Leaf Spot):
The mycelial plant body of the pathogen grows intercellularly and draws nutrition from the neighbouring cells by haustoria. With aggregation, the mycelium forms dense stroma from where long septate to non- septate, geniculate, hypophyllous conidiophores develop (Fig. 5.36C).
Conidiophores emerge in tufts by rupturing host epidermis. Conidia develop on conidiophores. Conidia are obclavate or cylindrical, septate, pale- brown and measure 30-50 µ x 5-6 µ (Fig. 5.36D).
The perfect stage of the pathogen is Mycosphaerella berkeleyii. The ascostroma is perithecial in nature, grows scattered, commonly along the lesion margin, partly embedded in the host tissue, broadly ovate to globose in shape, black, ostiolate and measured 84-140 µm x 70-112 µm.
Perithecia contain asci without paraphyses. Asci are cylindrical club-shaped with short stipes, bitunicate, 8-spored and measured 30-40 µm x 4-6 µm. Ascospores are 2-celled, slightly curved, upper cell somewhat larger, constricted at septum, hyaline and measure 11-19.5 µm x 3-3.8 µm.
Disease Cycle (Fig. 5.37):
The pathogen can perennate through conidia on diseased plant debris that remain in the soil, on shell of fruits and also on seeds. Perennating pathogen in all the above three places acts as a source of primary inoculum.
It favours high temperature ranges between 26°C to 31°C and high humidity. Continuous low temperature along with dew also favours infection. Infection takes place through stomata or by piercing the host epidermal cell.
Infection commonly takes place through upper epidermis and after entering the host tissue, the pathogen ramifies and by aggregation of mycelium, stroma develops.
With further development, the stroma creates a pressure and the conidiophores come out through ruptured epidermis. Conidia are developed on conidiophores. The conidia after detachment, disseminate through wind to the different regions of the same plant or to the different plants and cause further infection and thus help to spread the disease. This cycle repeats several times in the growing season.
Disease Management:
Following procedures are adapted to control or reduce the disease incidence:
A. Cultural Methods:
1. Sanitation:
Cleaning and destruction by burning the previous year’s plant debris, reduce the source of primary inoculum, thereby the disease incidence becomes reduced.
2. Crop Rotation:
Crop rotation for 2-4 years is a very good procedure to reduce the rate of infection.
3. Early Sowing:
It is a very useful procedure, where the plants mature early, thus the damage through disease becomes very less.
B. Chemical Methods:
1. Seed Treatment:
As the disease is seed- borne, seed treatment gives good result to control the disease. In this procedure, the seeds are properly separated from the shell and treated with 0.5% CuSO4 solution for 30 minutes before sowing. Dry dressing with Agrosan GN is also very much effective to reduce the source of primary inoculum.
2. Foliar Spray:
To reduce the source of secondary inoculum, foliar spray of chemicals is commonly used.
Different Chemicals used as Foliar Spray are:
a. Bordeaux mixture (4: 4: 50 or 5: 5: 50). It is used along with linseed oil (as sticker) at an interval of 15 days, which is much effective to reduce the disease to a significant level.
b. Other chemicals. Chemicals like Dithane Z-78 (0.2%), Dithane M- 45, Cosan and CuSO4 (15 and 25 lb/ acre), Fycol 8E etc., are very much effective but requires 5-6 spray.
c. Systemic fungicides. Bavistin, Benlate, Cercopin etc. are the systemic fungicides found to be very effective in controlling the disease. But, the chemicals are costly and it is advised to calculate the cost- benefit ratio before application.
13. Red Rot of Sugarcane:
The disease is prevalent in most of the sugarcane growing areas of the world in moderate to most destructive form. The disease is also common in sugarcane growing areas of India and appeared as epidemic in U.P. and Bihar during 1939-40 and 1946-47.
Host:
Saccharum officinarum L.
Symptoms:
The symptoms are visible on and inside stem and also on leaf.
On Stem:
In early stage of disease development, the affected green stem shows purplish colouration on rind. During the later part of rainy season or still later, the root primordia at the nodal region tends to convert into black dots, the acervuli. The infected plant shows the death of 3rd or 4th leaves and the entire crown becomes dry in severe attack.
Inside Stem:
The split open diseased stock shows red rot symptom of internal tissues. The infected tissue becomes dull red and interrupted by transverse white patches (Fig. 5.38A-C). The rind becomes shrunken during harvest with longitudinal cavity filled with profused mycelial growth.
On Leaf:
The symptom on leaf appears as elongated bright red lesions on the midrib of leaf blade and reddish patches on leaf sheath (Fig. 5.39). The lesions gradually spread throughout the length of midrib. The bright red lesion gradually changed with grayish centre, surrounded by dark reddish-brown margin. The margin become occupied by black dots, the acervuli of the fungus.
Causal Organism:
The causal organism i.e., the pathogen is Colletotrichum falcatum went. [Its Perfect stage is Glomerella tucumanensis (Speg.) von Arx and Muller., also named as Physalospora tucumanensis, an Ascomycetous member].
The fungus consists of septate, prostate much branched, thin-walled, hyaline hyphae growing intercellularly.
In nutrient-deficient condition, the germ tube develops appresorium at its tip in contact with hard surface like on host and also in culture medium. The appresoria are round, oval or irregular in shape, smooth, thick-walled and brown in colour.
The mycelium also develops thick- walled, round chlamydospores in old culture and also on host surface. The chlamydospores are the resting structures, and on germination they develop mycelium.
Conidia develop on hyphal tip and also inside the acervuli. The acervulus consists of a mass of short, closely packed conidiophores with numerous dark brown setae (Fig. 5.40A). The setae are also able to develop conidia-like conidiophores. The conidia are unicellular, hyaline, generally fulcate or sickle-shaped, with one end rounded and the other end pointed (Fig. 5.40B). The conidia measure about 20-40 µm x 5-7µm.
The sexual stage of the pathogen i.e., the perithecial stage has been reported from different countries like Australia, Brazil, China, India and Taiwan.
This stage was reported in India in 1952 (in culture) and also in 1953 (on leaf attached with plant). The perithecia remain embedded inside the host tissue except the projected ostiole. They are globose and measured between 85-250 µm x 100-260 µm.
Perthecia contain numerous hyaline asci that are intermingled with numerous hyaline paraphyses. The asci are hyaline, clavate and measured 70-90 µm x 13-18 µm containing 8 ascospores. Ascospores are unicellular, hyaline, straight or slightly fusoid and measured 18-22 µm x 7-8 µm.
Disease Cycle (Fig. 5.41):
Pathogen can perpetuate in infected seed sets, in soil, plant debris and collateral hosts. Inoculum is present in the form of conidia, setae, chlamydospores, appresoria, thick walled hyphae and ascospores.
The seed sets of diseased plant are the main source of primary inoculum. The infected seed sets have inoculum inside the host tissue or have dormant mycelium in the bud scales, leaf scars etc. These seed sets invariably developed infected shoots. The acervuli developed on the infected plants produce conidia that are transmitted commonly through irrigation or rain water and rarely by wind and cause further infection.
The inoculum from soil also infects the propagating stock and gradually the young plants. The infected plants develop acervulus on root primordia at nodes and also on leaves. The conidia developed from both the sources are dispersed by rain water or by wind and cause further infection.
The pathogen may survive in soil in the form of chlamydospores, setae, appresoria and also by thick-walled hyphae and causes further infection to the next crop, where there is a gap between the harvesting and next planting. But, the pathogen may remain within the host, where the crops are available in the field round the year.
The perithecia are not common in India, but play a significant role when present.
Collateral hosts, like Sorghum halepense, S. vulgare, Saccharum spontaneum, Leptochloa fiiliformis etc., serve a significant role in annual recurrence of disease.
Disease Management:
The disease can be controlled or reduced by the following methods:
A. Cultural Methods:
These include field sanitation, selection of seed sets, crop rotation and proper drainage in the field.
1. Field Sanitation:
The field should be free from previous year’s infected plant debris to reduce the primary source of inoculum.
2. Selection of Seed Sets:
The seed sets should be selected from healthy and vigorously growing plants.
3. Crop Rotation:
A long term rotation for 2-3 years help in eliminating the pathogen.
4. Proper Drainage:
Water-logging in the field increases disease incidence, so proper drainage is essential to reduce the disease incidence.
B. Physical Methods:
Heat treatment is effective to reduce the disease incidence:
1. Hot Water Treatment:
Seed sets are treated with hot water at 52°C for 18 minutes, to reduce the disease.
2. Hot Air Treatment:
Hot air at 54°C for 8 hours is found very effective to control the disease.
C. Chemical Methods:
Fungicides like Dithane Z-78, Blitox-50 and Carbendazim (0.1%) are used on standing crop and are found very effective to control the disease incidence.
D. Disease Tolerant Varieties:
The following disease tolerant varieties are recommended to cultivate — Co449, Co658, Col 158, Co1336, CoC671, CoL9 etc.
14. Downy Mildew of Bajra:
The Downy mildew or Green ear of bajra is available in temperate to tropical areas of the world, including Europe, U.S.A, Asia and Africa. In India, the disease was first reported by Butler (1907). With the introduction of high yielding varieties, incidence of the disease gradually increase and the loss becomes about 30%.
Later, in 1975, it devastated (about 100% loss) the kharif crop of hybrid bajra in Maharashtra and Karnataka of several thousand hectares of cultivation. About 45% loss in yield was also reported from North India.
Host:
Pennisetum typhoides SI apt & Hubb.
Symptoms:
The symptoms appear in two phases:
A. On seedling, and
B. On inflorescence.
A. On Seedling:
The infected seedlings turn pale yellow and become stunted due to shortening of internodes. Infection at an early stage causes stunting without any tiller formation. Infection at a later stage causes stunting with excessive tiller formation and thus the plant becomes bushy.
The leaves of the infected plant develop chlorotic streak on the upper surface and on the lower side below each streak. The downy whitish growth of the fungus appears on each streak. Later on, entire foliage becomes white due to downy growth of the fungus. Still later i.e., about 3 weeks of infection or later, the chlorotic streaks become brown. Ultimately, the death and drying of the entire seedling take place.
B. On Inflorescence:
The symptoms on inflorescence appear variously depending on the severity of infection. Different floral parts including glumes, stamens, pistils etc., are converted into green linear leafy structure of different sizes.
Symptoms are visible in 3 forms:
1. Cob length normal and lower half becomes converted into green leafy beard-like structure (Fig. 5.42A).
2. Cob length normal and entire cob is converted into green leafy, beard-like structure (Fig. 5.42B), and
3. Entire inflorescence becomes reduced and converted into leafy, beard-like structure without any grain (Fig. 5.42C).
Causal Organism:
The disease is caused by Sclerospora graminicola (Sacc.) Schroet. The fungus is an obligate parasite of Mastigomycotina.
The mycelial plant body is present in roots, stems, leaves and also in inflorescence of infected plants. The hyphae are coenocytic, branched, grow intercellularly and develop small bulbous haustoria to draw nutrition from the neighbouring cells. The hyphae grow freely in the mesophyll of leaves.
The sporangiophores develop in tuft from the tuft of hyphae accumulated below the stomata. The sporangiophores are broad with short hyphae, measuring 100-220 µm x 15-23 µm. The sporangiophores are initially unbranched, but become branched towards the upper side (Fig. 5.43A). Each branch ends in a sterigma bearing a single sporangium.
The sporangia are hyaline, smooth-walled, broadly elliptical and slightly pointed or papillate at the free end and measured 13-34 µm x 12-23 µm (Fig. 5.24B). Mature sporangia, after detachment, germinate in water and develop reniform, biflagellate zoospores. After a short period of swimming, they become encysted. On germination they develop infection hypha which infects the host.
The sexual stage, i.e., oosporic stage, is very common in India. Oospores are formed by the antheridium and oogonium develop in the infected tissue. Oospores are oval, 3-walled, 31- 39 µm and develop in large numbers inside the mesophyll (Fig. 5.43C). Oospores perennate for long period during unfavourable condition and with the onset of favourable condition, they germinate by developing 1-4 germ tubes (Fig. 5.43D).
Disease Cycle:
The disease is systemic. The pathogen is an obligate parasite and primarily seed-borne. The pathogen survives in nature and primary infection is caused by oospores. Oospores are produced in infected floral organs and leaves.
The infected parts, after falling on the ground, remain with the plant debris where the oospores perennate during winter. Oospores remain viable for 4 (in the soil) — 8 years (in host tissue). With the onset of favourable condition, the oospores germinate and infect the young seedling (about 9 days) systemically from the underground parts.
Oospores may be carried with seed during harvesting and thrashing. The oospore also serves as a source of inoculum to infect the young seedling.
The intercellular mycelium of the fungus also perennates in the embryo and inside the seed coat. It is presumed that the infection of inflorescence takes place by the intercellular mycelium and it progressively grows towards the apical region.
Secondary inocula, i.e., sporangia are formed on the infected regions favoured by range of temperatures between 15°-25°C. The disease is commonly seen in North India during August- September, when temperature and humidity favour the growth of the pathogen.
The sporangia are dispersed by wind, insect and water (both rain and irrigation water). They germinate on host surface in thin film of water to produce zoospores. The zoospores become encysted and form germ tube which infect the host.
Disease Management:
The disease can be controlled or reduced by the following methods:
A. Cultural Methods:
Various cultural methods used to reduce the disease incidence are:
1. Cleaning of previous year’s infected plant debris,
2. Deep ploughing and sun baking of soil,
3. Early planting,
4. Transplanting of seedling,
5. Removal of infected seedling i.e., rogueing,
6. Use of proper dose of nitrogen fertiliser, etc.
B. Physical Methods:
Treatment of seeds in hot water at 55°C for 10 minutes is useful to eliminate the seed-borne pathogen.
C. Chemical Methods:
Chemicals are used in 3 ways to reduce the disease incidence:
1. Seed Treatment:
Though the disease is seed-borne, seed treatment is very useful to control the disease.
(a) Seed treatment with Agrosan GN (0.1%) along with 0.4% Thiram (75% a.i) gives 50% reduction of the disease.
(b) Seed treatment with other fungicides, like Captafol and Thiram, is very useful to control the disease.
2. Foliar Spray:
Chemicals of different groups are found to be very effective as foliar spray.
These are:
(a) Mancozeb and Captan are found to be very effective to control the disease.
(b) Apron, Ridomil etc., are found to be very effective and used as seed treatment and/or foliar spray.
(c) Seed dressing treatment with Apron SD-35 (8 g/kg seeds), followed by foliar spray with Ridomil MZ-72WP is very useful to control the disease.
3. Root treatment. Seedling roots are dipped in water containing 2,000ppm Difolatan or Aureofungin for 1hr, just before transplanting gives good protection to adult plants.
D. Disease Tolerant Varieties:
WC-C 75, ICTP 8203, ICMH 451, Pusa 23, HB-5, NHB-10 and NHB-14 are the varieties recommended as disease-tolerant cultivars.
15. Powdery Mildew of Cucurbits:
The disease is observed throughout the world. In Asia, the disease occurs in India, China, japan, Iraq, Iran, Saudi Arabia, Israel, Singapore etc. The disease was reported first from Uttar Pradesh and Bihar.
The disease is very common in cucurbits, mainly in pumpkin and bottle gourd. Besides cultivated species, the disease also takes place in wild plants and also on other plants like potato, sunflower, lettuce, tobacco seedling, Sedum spurium, Sonchus asper etc.
Host:
Cucurbita maxima Duchesne, C. pepo DC. etc.
Symptoms:
Initially, the disease appears as minute white to grey coloured spots on leaves as well as on stems. With maturity the spots increase in size and give powdery appearance. Later on, entire leaf becomes covered by powdery mass of spores (Fig. 5.44). Towards the end of winter season, minute dark-brown pinhead bodies are unusually found to develop, intermixed with white powdery masses of spores.
These pinhead bodies are the ascostroma of cleistothecial type. During severe infection, the leaves fall off and fruits get deformed.
Causal Organism:
Powdery mildew is a widespread and serious plant disease in India and also in other countries. Both the organisms Erysiphe cichoracearum DC and Sphaerotheca fuliginea (Sc.) Poll, are responsible for the disease.
Regarding the occurrence of pathogens, different observations have been reported. Disease may be caused by one pathogen or by both the pathogens (but separately).
Disease Caused by One Pathogen:
1. Erysiphe:
It causes disease in Australia, Brazil, Bolivia, Canada, Egypt, France, Peru, Saudi Arabia, Singapore etc.
2. Sphaerotheca:
It causes disease in Australia, China, Czeckoslovakia, Greece, Iran, Japan, Malawi, Netherlands, Rumania etc.
Disease Caused by Both the Pathogens (but Separately):
Both the pathogens cause disease in different countries, dominated either by Erysiphe (Bulgaria, U.S.A. and Germany etc.) or by Sphaerotheca (Italy, India, Israel, Japan etc.) or by both in equal importance (Hungary, CIS (former U.S.S.R).
The Pathogens are Characterised as:
Erysiphe Cichoracearum DC:
Mycelial plant body is well-developed, generally evanescent, superficial with well-developed haustoria in host cells. Conidia are ellipsoidal or barrel-shaped and measure 24-44 µm x 14-26 µm, they develop in chain on unbranched and erect conidiophore (Fig. 5.45A). Cleistothecia are globose, scattered or gregarious and 90-130 µm in diameter with 10-20 µm thick wall.
Cleistothecia are surrounded by numerous mycelial appendages about 1-4 times larger than its diameter (Fig. 5.45B). Asci are ovate, rarely subglobose, more or less stalked, 12-25 per cleistothecium and measure 60-90 µm x 25-50 µm. Ascospores are 2 in each ascus (rarely 3) and measure 20-30 µm x 12-18 µm (Fig. 5.45C).
Sphaerotheca Fuliginea (Sc.) Poll:
Mycelial plant body is hyaline, but occasionally becomes brown with maturity, grows intercellularly and develops hauStoria to draw nutrition from the neighbouring cell. Conidia are ellipsoid to barrel-shaped, develop in long chain at the apex of simple and short conidiophore (Fig. 5.45D). The oidia measure 24-36 µm x 14-25 µm.
Cleistothecia are more or less round and usually 85 µm in diameter with 24 µm thick wall (Fig. 5.45E). Asci are elliptical to subglobose, 50-80 µm x 30-60 µm and develop singly inside cleistothecium. Ascospores are ellipsoid to nearly spherical, 16-22 µm x 12-19 µm and 8 in number in each ascus (Fig. 5.45F).
Disease Cycle:
The appearance of the disease in successive years is not clearly known. It is possible that the cleistothecia are the main source of inoculum. It might be possible that the conidia of fungi that are overgrown in summer in the hills are blown every year to the plains during winter and the reverse situation is observed during summer.
Jhooty (1967) reported that in Punjab (India), the vegetative mycelia and conidia survived on sheltered cucurbits and those inocula play an important role in annual recurrence.
Disease Management:
The disease can be controlled by the following methods:
A. Chemical Control:
1. Sulphur dusting. Like other powdery mildew, the disease can be controlled by sulphur dusting.
2. Karathane (0.2%) @ 1,000 litre/ha is usually recommended to control the disease (Mandloi and Khare, 1969).
3. Elosal (Wettable powder with 80% elementary sulphur) 0.5% also proved to be highly effective to control the disease.
4. Systemic fungicides, like Bavistin, Benomyl and Triozoles have been found to be highly effective to control the disease.
B. Disease Tolerant Varieties:
The varieties Huragola and Diguria of muskmelon are also very effective to resist against the disease.
16. Powdery Mildew of Grapevine:
The disease is observed throughout the world and the economic loss is much more in European countries and also in western USA. In Europe, the disease frequently caused complete destruction of grape cultivation in some places.
Afghanistan also suffers from this disease and in 1958, the loss was estimated about 50-80%. The disease is also available in India in moderate to severe form, mainly in Andhra Pradesh, Maharashtra, Gujarat and Tamil Nadu.
Host:
Vitis vinifera L.
Symptoms:
The symptoms appear on leaves, flowers and also on young berries. The disease first appears on both the surface of leaves as white powdery patches. Later oh’, similar symptoms are also visible on flowers and young fruits.
Infection on flowers and fruits caused much reduction in yield. Due to infection, the young berries become irregular in shape, dark in colour and develop cracks on wall. The plants become dwarf and also show wilting.
Causal Organism:
The fungus Uncinula necator (Schw.) Bur. is responsible for this disease. Mycelial plant body is very thin, septate and effused or forming patches. Conidia develop in long chain, ellipsoidal and measure 32-39 µm x 1 7-20 µm.
Ascocarp is of cleistothecial type and form very rarely. Cleistothecia are formed on the upper and lower surface of the leaves. Cleistothecia are globose, depressed, with 8-30 equatorially inserted appendages and measure 85-104 µm in diameter.
Appendages are 1-6 times more than the diameter of cleistothecium, thin-walled, septate, projected with a more or less helicoid apex. Asci are generally 4-6, but rarely 6-9, broadly ovate and measure 49-60 µm x 24-40 µm. Ascospores are commonly 4-7, elliptical to oval in shape and measure 15-34 µm x 10-12 µm.
Disease Cycle:
Mycelia and conidia of the pathogen overwinter in different plant parts like stem, diseased bud etc. Infection of developing buds occurs during the growing season by the perennating inoculum present inside the dormant bud. The shoot growing from the developing infected bud becomes covered by white mycelium.
Conidia developed profusely on the infected shoots, are readily disseminated by wind to the neighbouring plants. The ascocarps (cleistothecia) are also capable of overwintering. Rain-washed ascocarps are retained to the bark of vine.
The first formed leaves of shoots growing close to the bark are infected first by the ascospores released from the ascocarps which are retained in the bark. Thus, disease continues in the next season. The optimum temperature for mycelial growth and conidial production is 25°C which hamper greatly at 35°C and above.
Disease Management:
The disease can be controlled or reduced by the following methods:
A. Cultural Methods:
1. Proper Ventilation:
Maintaining free ventilation and reduction of shading of vine by trimming of shoots are useful to control the disease.
2. Pruning of Diseased Parts:
Pruning of diseased plant parts is very useful to reduce the disease incidence.
B. Chemical Control:
1. Dusting of sulphur. It is preferred in dry climate, where temperature is not high. Dusting of sulphur (300 mesh) is done generally in 3 times, 1st: when new shoots are 3-6 inches long, 2nd: during blossoming, and 3rd : after 40-50 days.
2. Wettable sulphur. It is used in areas with heavy rain fall.
3. Bordeaux mixture. Anderson (1956) recommended the application of Bordeaux mixture and fixed copper in controlling the disease.
4. Other effective fungicides. Person et al., (1994) recommended single application of fungicides, like Triadimefom (Bayle- ton), Triadimenol (Bayton), Myclobutanil, Etaconazole (Vangard), Penconazole and Flusiliazole between flowering and two weeks after shatter which are very much effective to control the disease.
C. Biological Control:
The disease can be controlled by using hyperparasite, Ampelomyces quisqualis.
D. Disease Tolerant Varieties:
Flame muscat is recommended as a disease tolerant variety in Bangalore, Karnataka; as reported by Sohi and Sridhar, 1976.
17. Bacterial Leaf Blight of Rice:
The bacterial leaf blight of rice was first observed in 1884 by farmers of Fukuoka in Japan. By 1926, the disease became widespread in the different regions of Japan, mainly in the south west and north east regions.
In Japan, the disease is known as ‘white withering disease’. Ishiyama (1922) established that the disease was caused by bacteria. In India, the disease was first recorded from Khopoli of Maharastra in 1951. Later, Srinivashan et al., (1959) reported that the disease was caused by Xanthomonas oryzae.
Due to introduction of high yielding varieties and frequent use of nitrogen fertiliser the disease was commonly observed in different parts of India. In India, Srivastava and Rao (1966) estimated the loss ranges from 6-60% depending on the variety of rice.
Now the disease is commonly recorded in Asian countries like China, India, Indonesia, Japan, Sri Lanka, Thailand and Philippines. Mew et al., (1993) reported that it is one of the most destructive diseases of rice in Asia.
Host:
Oryza sativa L.
Symptoms:
The symptoms are visible at all stages of development and in all parts of rice, except root.
The symptoms appear as water soaked tiny spots on the margin of a mature lower leaf of seedling. With maturity, the leaves become yellow and gradually dry out. On leaf blade, lesions generally appear on the margin or any other region and a few are also formed at the apex as water soaked stripes. The lesions gradually enlarge both in length and width and the leaf becomes yellow within few days (Fig. 5.47A, B).
Glumes of immature grains also become infected showing discoloured spots surrounded by water-soaked tiny spots. The infected grains mature earlier than the healthy one.
The most harmful phase of disease is the Kresek or wilting symptom, which was first described in Indonesia. In tropical countries, the apex of the leaves of seedlings is trimmed off before transplanting to reduce the size for proper handling. The infection starts through the cut ends and within one or two weeks, the symptom appears as water-soaked green spots on the leaf.
Gradually the leaves become greyish-green, roll along the midrib and then withering of entire leaf (including leaf sheath) takes place. The bacterium can move through xylem vessel and infect the other leaves, thereby the entire plant becomes dead.
(When a few leaves wilted and are seen to float on water, it is called Kresek or wilting symptom; but when complete death of entire plant takes place it is called Hama lodoh.)
In comparatively resistant varieties, just inside the margin of the leaf blade, the symptom appears as yellow stripes without showing any necrosis. Milky or opaque bacterial drops may be observed in the early morning on the young lesions.
Collateral Hosts:
Leersia oryzoides and Leersia oryzoides var japonica are well-known collateral hosts, effective only in Japan.
Causal Organism:
The disease is caused by Xathomonas campestris pv. oryzae (Ishiyama) Dye. Earlier it was known as X. oryzae, Bacillus oryzae or Pseudomonas oryzae. It is a Gram- negative, non-spore forming, rod-shaped bacterium with round ends measuring 1-2 µm x 0.8-1 µm with monotrichous flagellum of 6-8 µm.
The bacterial cells are capsulated and grow in mass. In vitro culture, the colonies show yellowish in colour. The optimum temperature for growth of bacterium is 25-30°C and the thermal death point is 53°C.
Disease Cycle (Fig. 5.48):
The disease cycle of bacterial leaf blight of rice (BLB) is not clearly known. The source of primary inoculum and the initiation of disease also vary in different countries and even in different places of the same country. The various prepositions regarding disease cycle are based on the different informations published by the plant-scientists of different countries.
The BLB is a vascular disease. The bacterium can perpetuate in different ways, such as through soil, seeds, wild grasses, diseased stubbles and straw.
Soil:
The bacterium may survive in soil for 1-3 months, depending on soil acidity and moisture. Due to short period of survival, the soil inoculum does not play any role in the transmission of disease to the next season.
Seeds:
The bacterium can survive for varying periods in infected seed and is transmitted to the next crop. According to Kauffman and Reddy (1975), the bacterium remains viable in infected seed only for two months. More recently, Debnath (1992) reported that, in India, the bacteria can propagate from season to season through infected seeds.
After sowing of infected seeds, the bacteria grow on roots and shoot surfaces which act as primary inocula. The bacteria present in palea and lemma ooze out into soil water during germination and through flooding, the bacterium is transmitted by submergence of seedlings in nursery beds.
The bacterium then establishes itself in new host, increases in number by multiplication and initiates further infection during favourable condition.
Wild Grasses:
The role of several weeds as the source of primary inocula suggests a new line of interest. Out of several weed hosts tested in Japan, Leersia oryzoides and Leersia oryzoides var japonica are considered as active natural hosts. The role of wild grasses as the source of primary inocula is yet to be confirmed.
Diseased Stubbles and Straw:
In Japan, bacterial population with high intensity is found in infected stubbles, just after the harvesting of the crop. But after 1-2 months, the bacteria die along with the decomposing stubbles in both warmer and cooler regions.
In India, the bacterium can survive in diseased stubbles in double- cropped areas, but not in such areas having single crop. In other countries, like Sri Lanka and Thailand, the bacterium surviving in diseased stubbles can develop primary inoculum for further infection. The bacterium will die within 20 days if the stubbles are ploughed down.
In Japan, the bacterium can survive up to next spring in diseased straw, if protected from rain; but die if it remains exposed. Diseased straw used in nursery bed or near an irrigation canal acts as the source of primary inoculum.
In some regions of India, ratoon in lowlands with optimum soil moisture is the source of primary infection.
The bacterium enters the host through wounds made by the chafing and striking of leaves by wind, either through the cut ends (by artificial cutting during transplanting) of seedling leaves, or through broken roots during transplanting or through natural opening like hydath- odes and stomata.
After entering the leaf, the bacterium multiplies and moves in both upward and downward direction. The symptom is visible within about 10 days of artificial inoculation. The exudates of bacteria that develop on the leaf surface are dispersed by rain.
The dispersed bacteria in contact with other leaves may cause infection or they may fall in water and carried to the other fields during irrigation and may cause further infection. In course of time, the inflorescence gets infected and will develop the infected seeds.
The infected plants are cut out for harvesting, whose upper part, after drying, becomes the diseased straw, and lower cut end is the diseased stubbles. Then the disease may continue through the infected seeds, disease straw and/or through diseased stubbles.
Disease Management:
The disease can be controlled or reduced by the following methods:
A. Cultural Methods:
1. In Japan, the nursery beds and fields are kept from being deeply flooded to reduce the disease. But this method is doubtful in tropical countries.
2. In India, previous year’s plant debris should be burnt to destroy the bacteria present in the infected straw.
3. In Philippines, the elimination of ratoon rice and volunteer plants has been recommended to reduce the source of primary inoculum.
4. Preparation of nursery beds in disease- free areas is done to restrict the inflow of primary inoculum into the main field which might reduce the disease incidence.
5. Injury is a prerequisite for entry of the bacterium in the host. As pruning of the apical region is a common practice in many parts of India, it is imperative that pruning should be avoided to resist the entry of the pathogen.
6. The time of application of nitrogen fertiliser influences the disease incidence.
Application of nitrogen fertiliser at tillering stage increase the disease incidence, but similar application — at the time of transplanting or during flower initiation — reduces the disease incidence.
B. Physical Methods:
1. Soaking the seeds in water for 12 hours, followed by hot water treatment for 30 minutes at 52-54°C, reduce the disease incidence.
C. Chemical Methods:
(a) Seed Treatments:
As the disease is seed- borne, various seed treatments are followed to reduce the disease incidence:
1. Soaking the seeds for 12 hours in a mixed solution of 0.05% Ceresan and 0.02% Agrimycin, followed by hot water treatment for 30 minutes at 52-54°C.
2. Soaking the seeds for 8 hours in 0.01% Ceresan and 0.6 gram of Streptocycline in 5 gallons (1 gallon = 4.55 it) water.
3. Soaking the seeds for overnight in Streptocycline solution at 100 ppm concentration.
4. Soaking the seeds for 16-24 hours in decinormal nitric acid solution, followed by washing in tap water and finally drying in sun.
(b) Foliar Spray:
Spraying of different chemicals is also effective to control the disease:
1. Foliar spray of 60-120 ppm Chloramphenicol, an antibiotic, in combination with phenyl mercury acetate at 10-20 ppm has been found effective in Japan.
2. Foliar spray of a mixture of 100 ppm each of Cellucidin and Dithianone is also very effective.
3. According to Singh (1 975, 76), 5 sprays of Agrimycin mixed with Copper oxy- chloride at 12 days’ interval is effective to control the disease.
(c) Other Methods:
1. Chlorination of irrigation water help in reducing the disease.
D. Disease Tolerant Varieties:
In India, many disease tolerant varieties are recommended for cultivation. These are W 259 and W 348, Tainan 3 and Chai- nung 292 etc.
18. Citrus Canker:
Citrus canker is stated to have originated from China and now the disease becomes widespread in all the citrus growing regions of the world. Sometimes, the disease becomes so severe in USA that mass eradication of diseased plants or the entire orchards has to be undertaken to control the disease. It also causes serious damage in the citrus fruit growing regions of India, China, Java and Japan.
Host:
Citrus aurantium L. (sweet orange) and other Citrus spp.
Symptoms:
The disease affects all parts of the plant, except the root, but the most susceptible regions are the leaves, fruits and twigs:
(i) On Leaf:
Lesions on leaf first appear as small, watery, round, translucent spots. The lesions are raised and become yellowish brown in colour. They appear first on the lower surface and then gradually spread on both the surfaces of disease, the surface of the spots becomes greyish and finally ruptures in the centre showing rough, corky and crater-like appearance.
The size of the spots ranges from 1 mm to 1 cm in diameter. Lesions occurring on petioles cause premature defoliation.
(ii) On Fruits:
Lesions on fruits are almost similar to those of leaves, except that the yellow halo and crater-like depression in the central region are more prominent. Lesions often coalesce and form very irregular patches of rough and scabby raised areas (Fig. 5.49C). The injury takes place only on the skin and causes no effect on the pulp or juice.
(iii) On Twigs:
Lesions on stem are like those of leaves, but they coalesce to form elongated, rough and scabby regions (Fig. 5.49A). On larger branches the lesions are irregular, rough and more distinct.
Causal Organism:
The disease is caused by Xanthomonas axonopodis pv. citri. Earlier, it was known as Pseudomonas citri, Xanthomonas citri or X. campestris pv. citri.
The bacterium is Gram-negative, rod- shaped, aerobic and capsulated, having single polar flagellum (monotrichous). They grow in chains and measure 1.5-2.0 µm x 0.5-0.75 µm. Colonies on beef extract agar are circular, slightly raised, glistering and amber yellow to straw yellow in colour.
Disease Cycle (Fig. 5.50):
The bacterium enters the host through stomata, lenticels and also through wounds caused by insects, movement of thorns etc. The bacterium multiplies rapidly in the intercellular spaces, thereby dissolves the middle lamella and establishes themselves in the cortical region. Mild temperature (20-30°C) and wet weather with uniform rain are most suitable conditions for their infection.
The bacterium does not survive in the soil or on infected plant parts fallen on the ground, due to antagonistic action of other soil-microorganisms. Infected twigs with old lesions on the tree are the main source of perennation of the bacterium.
The bacterium then disseminates by wind, rain and/or by insects like Citrus leaf miners (Thosconyrsa citri and Phyllocnistis citrella) and causes infection to the new healthy plant. However, the disease is commonly disseminated into the new locality through the infected nursery stock.
Disease Management:
The disease can be controlled or reduced by the following methods:
A. Cultural Methods:
1. Sanitation:
Pruning of the infected parts during dry season and destruction of the affected plants by burning reduce the source of primary inoculum.
2. Quarantine Measure:
Strict quarantine measures should be adopted to restrict the dissemination of bacteria from one country to other even from one locality to others of the same country.
3. Disease-Free Nursery Stock:
Disease can be controlled at a significant level by using disease-free nursery stock.
4. Vigour of the Plant:
The vigour of the plant should be maintained by application of proper amount of fertiliser and irrigation.
B. Chemical Methods:
1. Fungicides:
Spraying fungicides like Lime-sulphur and Bordeaux mixture is often very useful to control the disease.
2. Antibiotics:
Spraying of antibiotics like Phytomycin (2500 ppm) or Streptomycin (500-1000 ppm) is very effective to control the disease.
C. Other Substances:
Spraying of Neem cake (160 lb/acre) is very effective to control the disease. 50 lbs (1 lb = 453.6 grams) cake is soaked in 20 gallons (1 gallon = 4.55 It) of water and kept for a week to decompose. It is then sprayed on the plant without filtration. 2 sprays at 2 weeks’ interval during August-September are very effective.
19. Tungro Disease of Rice:
Tungro (degenerated growth) is the most serious disease of rice known in the Philippines since middle of 20th century. It is of unknown etiology. In 1965, Rivera and Ou was the first to report that the causal agent is a virus.
Now, the disease occurs severely in South and South East Asia, from India to Philippines, along with some neighbouring countries. In India, the outbreak of the disease occurred in 1965 in the eastern region and most recent outbreak of the disease took place in West Bengal in 1981. Earlier, the disease was known as penyakit merah (Malaysia) and yellow orange leaf (Indonesia).
Host:
Oryza sativa L.
Symptoms:
The symptoms of the disease are visible as stunting of plants and discolouration of leaves (Fig. 5.51 A). The symptoms may vary in different cultivars. Some cultivars show prominent clearing of veins in discoloured infected region of the leaf, but in others it remains indistinct. The cultivars also show various shades of orange or yellow colouration.
Some small necrotic and rusty spots may develop in the discoloured zone of older leaves. It shows the symptoms like stunting growth, less number of tillers, reduction in the length and number of panicle, number of spikelets, weight and starch content of grains — thereby the total yield becomes much reduced.
The RTSV is not able to show any clear symptoms in most cultivars. The milder symptoms caused by RTBV alone become intensified in the presence of RTSV and show typical (yellowing and stunting) symptoms.
The disease is caused by two viruses:
(a) Rice tungro bacilliform virus (RTBV), and
(b) Rice tungro spherical virus (RTSV).
(a) RTBV (described as Badnavirus):
It has bacilliform capsid (Fig. 5.51 B), measuring 130 nm x 30 nm and is made up of single species of coat protein of 36K. It contains single molecule of double- stranded circular DNA of 8.3 kbp.
(b) RTSV (described as Waikavirus):
It has isodiametric capsid (Fig. 5.51 B), measuring 30 nm in diameter and is made up of 2-3 species of coat protein. It contains single-stranded RNA of about 10kbp.
Disease Cycle (Fig. 5.52):
The disease is caused by both the viruses RTBV and RTSV. They are able to multiply independently and are transmitted by the green leafhoppers (Nephotettix virescens and N. nigropictus) and other leafhoppers. The N. virescens is the principal vector of the disease where adults are more efficient than the youngers.
It can transmit both viruses together after feeding on infected plant. The RTSV is easily transmitted by both the green leafhoppers (N. virescens and N. nigropictus), but RTBV is transmitted by N. virescens only in presence of RTSV. The vectors move during night, but remain with the plant during day.
Disease Management:
The disease can be controlled or reduced by the following methods:
A. Cultural Methods:
1. Sanitation:
The stubbles should be removed by ploughing to reduce the amount of inoculum in the field.
2. Transplantation Time:
The proper time of transplantation should be selected to avoid vector pressure during early stage of growth of seedlings. The vector pressure becomes more in the rainy days of April and May.
3. Location of Seed Bed:
The location of seed bed should be at a distance of more than 10 m from standing crop.
B. Chemical Control:
1. Just after emergence of seedling, the application of Foratox 10C @ 1.75 kg/ha or Furadan 3G @ 1.5 kg/ha in the seedbed keeps the area free from vectors.
2. Spraying of insecticides like Carbofuran and Cypermethrin at 0.1% conc. gives significant reduction of vector population.
3. Application of Carbofuran @ 1 kg/ha in the root zone causes much reduction of vector population and is better than the spray treatment. Application of BPMC and Bendiocarb in the root region also gives significant control of vector population which is almost equivalent to the spraying of Carbofuran.
20. Tobacco Mosaic Virus:
This is the best known of all virus diseases of plants and distributed throughout the world. The virus affects more than 150 genera of dicotyledonous plants of which tobacco, tomato and cucurbits are very important. The virus does not affect any monocotyledonous plants.
The disease causes great economic loss by reducing both the quantity and quality of tobacco. It does not develop any external symptom on the affected grapes and apples.
Adolph Mayer (1886) was the first to point out the mosaic pattern symptom on leaves of tobacco plants. Iwanowski (1892) demonstrated that the infecting principal can pass through bacteria-proof filter and able to induce mosaic disease in healthy plants. A few years later, Beijerinck (1898) proved the existence of a virus in the affected plant as described by Mayer.
Host:
Nicotiana tabacum L.
Symptoms:
The symptoms are of different types showing various degree of chlorosis, mottling, curling, dwarfing and blistering of leaves (Fig. 5.53A, B); dwarfing of the entire plant and discolouration of flowers. The variation takes place due to variation of strain of virus and the variety of plants affected.
The symptoms on young leaf appear as faint chlorotic circular lesions with gradual vein clearing. With gradual development, the characteristic systemic mosaic symptom appears. With maturity, the leaf shows abnormal dark-green spots which develop into crumpled blister-like areas of irregular outline w and rest of the tissue becomes more or less chlorotic.
During severe infection, the leaves become narrow, wrinkled and thin with severe malformation. The infected young plants become much more stunted than the older one. The disease causes partial pollen sterility but is rarely fatal to the host.
Causal Organism:
The pathogen or causal organism of this disease is Tobacco Mosaic Virus (TMV), recently designated by the generic name Tobamovirus, consisting of a number of viruses including Tobacco Mosaic Virus, Tomato Mosaic Virus etc.
For details of TMV structure see Fig. 2.29.
Disease Cycle (Fig. 5.55):
The TMV survives in infected plant debris, on surfaces of contaminated seeds, on contaminated seedbed cloths, tobacco refuses from warehouses, cigars, cigarettes, pipe and chewing tobacco which form the source of primary inoculum (Fig. 5.54).
The virus is dispersed from plant to plant by mechanical transfer during transplanting, through field operations and implements and contact by man during cultivation. The virus initially infects wounded tissue of the seedling in seedbed or the transplanted plant in the field. It develops systemic infection in all plants.
After invading wounds it infects the parenchyma cell. The virus then multiplies and gradually moves from cell to cell through the plasmodesmata and, after reaching the phloem, it moves systematically and infects the whole plant. In the cytoplasm of the host the virus remains as a crystalline body. The infected plant again serves as the source as the primary inoculum, either directly or indirectly, for the next season.
Disease Management:
The disease can be controlled or reduced by the following methods:
A. Cultural Methods:
1. Previous year’s plant debris should be collected, removed and burnt outside, in the field.
2. Infected plants should be eradicated and burnt to check further spread of the disease.
3. Seed beds should be located far away from the tobacco warehouses.
4. Seed beds should be free from tobacco refuse.
5. Proper care should be taken to avoid the chance of contamination through cultivation implements.
6. After weeding, hands should be thoroughly washed with soap in running water.
7. Susceptible hosts and weeds should be destroyed thoroughly.
8. Chewing or smoking of tobacco of any kind should be avoided to reduce the source of primary inoculum.
B. Physical Control:
1. Soil of seed bed should be sterilised by hot water or steam.
2. According to Hare and Lucas (1959), infection by TMV is inhibited by whole milk. For the above, whole milk used by spraying on seedling before transplanting and washing of hands by dipping in
milk — both reduce the spread of disease. This method is now highly recommended in some regions of USA.
C. Chemical Control:
1. Spraying of tannic acid (0.5-1.0% concentrations) at 10 days’ intervals, starting from 20 days after planting of tobacco, gives good control over the disease.
D. Biological Control:
1. Cross-protection of plants from TMV has been reported. The plants are first inoculated with a milder strain of TMV, thereby the plants are able to resist the action of the virulent strain.
E. Disease Tolerant Variety:
1. Use of tolerant variety is the best way to protect the crop from TMV. Tolerant varieties like TMVRR-2 and TMVRR-3 are developed by Central Tobacco Research Institute (CTRI) at Rajamundry.