In this article we will study about the tissue systems seen in plants:- 1. Epidermal (Dermal) Tissue System 2. Fundamental or Ground Tissue System.
Contents
Study of Epidermal (Dermal) Tissue System:
This system consists of the outermost skin or epidermis of all plant organs. It represents the region of contact between the plant and the environment. It is primarily a protective tissue.
Some uncommon features are discussed below:
1. Multiple Epidermis:
Material:
Leaves of Ficus benghalensis, Nerium; epiphytic roots of Wanda (Orchid).
Cut T.S. of the materials, mount in 50% glycerine and observe under microscope.
In Ficus sp. leaf the upper epidermis is multiple or multi-seriate. The outermost layer of cells are small, compactly set with cutinised outer wall and cuticle. The cells of the inner layers are larger and with small intercellular spaces. All the cells are tabular and colourless. Cystoliths are often present.
In Nerium leaf both upper and lower epidermal layers are multiple, usually with three layers of cells. In the lower epidermis there are depressed areas at regular intervals containing stomata and hairs and here the epidermis is single-layered. Cells are compactly set, colourless and isodiametric. Uppermost and lowermost cell layers are cutinized on the outer surface and have cuticle.
In the aerial root of Vanda the multiple epidermis is known as velamen. It is a spongy tissue and consists of a few layers of compactly set non-living cells having a silvery outer coating. The cell walls are porose and are variously thickened by spirally or reticulately arranged fibres. The cells are empty and remain full of air in dry weather and full of moisture during rainy season.
2. Epidermis of Saccharum Officinarum (Sugarcane) (Fig. 5.1 D):
Peal off the epidermal layers of sugarcane leaf, mount in 50% glycerine and observe under microscope. The epidermal cells are rectangular and elongated in the direction of the long axis, as is found in
most monocotyledonous stems and leaves.
In sugarcane leaf there are also occasional pairs of small rectangular cells one of which is called cork cell and the other silica cell. Cork cells have suberized walls and silica cells have silica particles impregnated on the walls.
Cut T.S. of sugarcane leaf and note the presence of rods and granules on the cuticle.
3. Epidermis of Aloe and Musa Leaves (Fig. 5.1 A, B):
Cut T.S. of the leaves and note that cuticle has projected into radial walls of the cells of epidermis as pegs.
4. Epidermis of Pine Needle (Fig. 5.1 C):
Cut T.S. of Pine needle. Stain with aniline sulphate solution. The epidermal cell walls turn bright yellow indicating the presence of lignin.
5. Buffliform Cells:
Material:
Leaves of Bamboo or Wheat.
Cut T.S. of the leaves, mount in 50% glycerine and observe under microscope. Note the presence of bulliform cells in the upper epidermis. They occur in groups at regular intervals and have a fan-like appearance as the central cell is the largest and the lateral cells are progressively smaller. The cells are colourless, thin-walled and cutinized on the outer side.
6. Structure of Stomata:
Material:
Leaves of Basella sp.
Cut T.S. of the leaf, mount in 50% glycerine and observe under microscope. Observe the stomata in the epidermis. Each stoma has a narrow aperture bounded by two guard cells. The guard cells have dense cytoplasm with chloroplasts and unevenly thickened walls.
Inner walls are thicker than the outer walls. The thickening on the inner wall becomes beak-like with an outer edge and an inner edge. Just below the stomatal opening lies a prominent air-cavity which is called sub-stomatal chamber.
For studying the surface view of stomata, peel-off the lower epidermis, mount in 50% glycerine and observe under microscope. In surface view the stomatal aperture looks elliptical and the guard cells look kidney- or bean-shaped. The epidermal cells lying in direct contact with the guard cells are called subsidiary or accessory cells.
7. Study of Types of Stomata (Fig. 5.2):
Materials:
Leaves of Clematis, Brassica or Nasturtium (Rorippa), Dianthus, Gardenia or Ixora and any grass.
Peel-off the lower epidermis of the leaves, mount in 50% glycerine, and observe under microscope.
A number of types of stomata have been recognised by Metcalfe and Chalk in angiosperms on the basis of their method of development, relation with adjacent cells as well as occurrence and number of subsidiary cells.
(A) Ranunculous or anomocytic type:
Stoma remains surrounded by a limited number of cells which are not distinguishable from other epidermal cells. Hence the subsidiary cells may be said to be absent. It is the anomocyclic or irregular-celled type, e.g. Clematis sp. (Fig. 5.2A).
(B) Cruciferous or anisocytic type:
Stoma is surrounded by three subsidiary cells of which one is much smaller than the other two. It is called anisocyclic or unequal-celled type e.g. Brassica, Nasturtium, etc. (Fig. 5.2B).
(C) Caryophyllaceous or diacytic type:
Stoma is surrounded by two subsidiary cells whose common wall is at right angles to the guard cells. This is the di-acyclic or cross-cell type, e.g. dianthus sp. (Fig. 5.2C).
(D) Rubiaceous or Paracytic type:
In this type the stoma has on either side one or more subsidiary cells which lie parallel to the long axis of the aperture or guard cells. This is called the para-cyclic or parallel-celled type, e.g. Gardenia, Ixora, etc. (Fig. 5.2D).
(E) Graminaceous type:
The guard cells are dumb-bell shaped with narrow middle portions and bulbous ends. The subsidiary cells are more or less triangular and lie parallel to the long axis of the aperture. The stomata are arranged in longitudinal rows, e.g. all grasses (Fig. 5.2E).
8. Study of Epidermal Hairs (Fig. 5.3):
Various types of hairs or trichomes originate from the epidermis. Scrape the surface of the plant organ (stem or leaf) with a razor or safety razor blade and mount the scraped material in 50% glycerine and observe under microscope. Staining may be done with 1% aqueous eosin solution.
Hairs may be unicellular or multi-cellular. Multicellular hairs have two parts — the basal part, called foot, remains embedded in the epidermis and is usually made of small cells and the apical part, called body, remains projecting above and is normally made of elongated cells.
Hairs of the following plants may be studied:
Lantana camara (stem and leaf) — sharp-pointed, multicellular hairs.
Helianthus annuus (leaf) — multicellular, uniseriate, soft, silky hair.
Lycopersicon esculentum (leaf) — multicellular, uniseriate hairs.
Mimosa pudica (leaf and stem) — multicellular hairs, cells forming base are scale-like.
Solarium verbascifolium, Platanus sp. and some of Mimosa sp. — multicellular, dendroid (tree-like) hairs.
Hibiscus rosa-sinensis and species of Malvaceae — stellate hair.
Nicotiana plumbaginifolia and Viola sp. — glandular hair or colleter having a rounded apex which is made up of a number of small cells.
Urtica dioica, Fleurya interrupta (stinging nettle):
The broad base is embedded within the epidermal cells and the body looks like a fine capillary tube with a pointed end.
Root hairs of seedlings (Pisum sativum, Brassica nigra etc.):
Hairs arise from a particular region of the roots which lie somewhat behind the tip of the root. The epidermis of this region is made of alternating short and long cells. The hairs arise as direct prolongations of the short cells. They are always unicellular. The nucleus lies at the tip of the hair.
Study of Fundamental or Ground Tissue System:
This tissue system is heterogeneous in nature consisting of diverse types of cells and includes all the tissues excepting the epidermis and the vascular bundles. The ground tissues occurring outside the stele are extrastelar ground tissue, or cortex and those occurring internal to the stele form the intrastelar ground tissues.
1. Cortex:
The cortex is highly variable and consists of parenchyma, collenchyma and sclerenchyma cells. The term hypodermis is applied to the zone of collenchyma or sclerenchyma which lies just below the epidermis and acts as a protective or supporting tissue. Cortex also contains resin-ducts, laticifers, oil cavities, sclereids etc.
2. Endodermis (Fig. 5.4):
Material:
Rhizome of Marsilea, root of Cicer, Colocasis. Zea, Smilax, stem of Helianthus, Cucurbita.
Cut T.S. and L.S. of rhizome of Marsilea and only T.S. of the other materials, the sections may be stained with aniline sulphate, chlor-zinc-iodine, phloroglucin and HCl and also KOH solution to get lignin and suberin reaction. Mount in 50% glycerine and observe under microscope.
The endodermis is the innermost layer of cortex, one-layered and composed of modified parenchyma cells which are usually barrel-shaped, compactly set and have no intercellular spaces. The most distinctive feature of endodermis is the presence of the Casperian strips or bands made of a waxy substance like suberin in the form of bands or strips on the radial and tangential walls. The strips also have some lignin.
Rhizome of Marsilea:
Note the occurrence of two endodermal layers — first one just outside the vascular bundles and the other delimiting the pith from the vascular tissues. The latter is called the inner endodermis. Note also the barrel-shaped cells which are compactly set with casperian strips in both T.S. and L.S.
Root of Cicer:
The casperian strips are extended to thicken the entire radial walls.
Root of Colocasia:
Same as in Cicer.
Root of Zea:
The casperian strips has become extended to thicken the radial and inner tangenitial walls. Occasional cells are entirely thin-walled. These are called passage cells. This condition may also be found in old Colocasia roots.
Root of Smilax:
All the walls of the endodermal cells are thickened and the lumens of cells are very narrow. Passage cells or transfusion cells are present. Endedermis with only casperian strips are called thin-walled or primary endodermis. Those with thick radial and tangential walls are known as thick-walled or secondary endodermis.
Stem of Helianthus and Curcurbita:
A typical endodermis with casperian strip is absent. But the innermost layer of cortex is made of compactly set barrel-shaped cells which contain abundant starch grains. This layer is called starch sheath. The presence of starch can be confirmed by iodine test.
Pericycle:
Pericycle is regarded as the outermost layer of stele. It lies between endodermis and vascular bundles. It may be single-layered or multi-layered and parenchymatous or sclerenchymatous, or both. It is absent in many angiospermic stems but universally present in roots.
Pith or Medulla:
This is the main internal ground tissue and occupies the central part of roots and stems. However, in many dicotyledonous roots, it is absent. The cells are parenchymatous with copious intercellular spaces. In many cucurbits and grasses the pith is hollow.
3. Ground Tissues of Leaves:
The ground tissues of leaves are called mesophyll tissues. In dicotyledonous leaves (dorsiventral) mesophyll tissue is differentiated into two types — large columnar cells arranged at right angles to the epidermis — called palisade parenchyma, a fid isodiametric cells with copious intercellular spaces — called spongy cells — occupy the middle region. In monocotyledonous leaves (obi-lateral) only spongy parenchyma is found. All mesophyll cells are chlorenchymatous.
Study of Vascular Tissue System:
The vascular tissue system is composed of the two complex tissues, xylem and phloem, which constitute discrete strands called the vascular bundles. In the stem of gymnosperms and dicotyledons a few layers of meristematic cells are present in between xylem and phloem. This is called cambium.
Types of Vascular Bundles (Fig. 5.5A, B, C, D, E, F):
Materials:
Stems of Helianthus, Zea, Cucurbita, Dracaena, Dryopteris or Pteris; roots of Cicer or Pisum.
Cut T.S. of the materials, make a suitable double staining to distinguish xylem and phloem and observe under microscope. For ready observation, mount in 50% glycerine.
1. Collateral Open Vascular Bundle:
Stem of Helianthus:
Xylem and phloem are placed side by side along the same radius, phloem on the outer and xylem on the inner (towards the pith) side. A strip of cambium is present in between xylem and phloem.
2. Collateral Closed Vascular Bundle:
Stem of Zea:
The arrangement of xylem and phloem is the same as in Helianthus, but cambium is absent.
3. Bicollateral Open Vascular Bundle:
Stem of Cucurbita:
The middle portion of the bundle is occupied by xylem and this is flanked on both inner and outer side by strips of cambium, and then two patches of phloem.
4. Concentric Vascular Bundle:
Here one kind of vascular tissue is completely surrounded by the other kind.
This is of two different types:
a. Amphivasal or Lepto-centric vascular bundle — stem of Dracaena:
Here phloem lies at the centre and xylem surrounds it. Amphioribral or hadrocentric vascular bundle. In the stem of Pteris and Dryopteris; Here xylem lies at the centre and phloem remains surrounding it.
b. Radial Vascular Bundle — Roots of Cicer, and Pisum:
Xylem and phloem are present separately along alternating radii of a circle and are intervened by non-vascular tissues.
Nodal Anatomy — Leaf and Branch Traces and Gaps:
Vascular supplies to leaves and branches (axillary buds) are known as leaf traces (foliar traces) and branch traces (ramular traces). In ferns, gymnosperms and angiosperms just above the traces parenchyma cells, instead of vascular elements, are found up to a certain distance. These parenchymatous zones are called leaf gaps and branch gaps (Fig. 5.6). Such gaps are absent in lower vascular plants such as Lycopodium, Equisetum, etc.
In angiosperms the number of leaf traces may be one, three, five or more. Dicotyledons with one leaf trace and one leaf gap are called unilacunar, those with three traces and gaps are trilacunar, and those with many traces and gaps are multilacunar. In phanerogams the number of branch traces and gaps is usually two. However, in some plants, there are only one or more than two branch traces and gaps.
Material:
Twigs of Hibiscus, Gardenia, Quisqualis, Nerium and Solanum.
For proper demonstration of nodal anatomy, microtome section, both L.S. and T.S. of nodal region are necessary. These are to be suitably double-stained, as for example with safranin and light green.
For ordinary classwork, cut T.S. of the nodal region at levels as shown in Fig. 5.6 (a). Make L.S. also, if possible. Stain suitably (double-staining) or mount in 50% glycerine and observe under microscope.
The central core of the axis — including all tissues from pericycle up to centre — form stele. It is, therefore, composed of pericycle, vascular tissues as well as pith and medullary rays, if present. In the vascular plants wide range of variation in stellar structure is found.
Materials:
Stems of Selaginella kraussiana, or Psilotum nudum (triquetrum), Lycopodium clavatum, Marsilea minuta, Salvinia natans, Dryopteris filex-mas, Helianthus annuus, Zea mays, and Roots of Pisum sativum.
Cut. T.S. of the materials, make suitable double stained preparations, mount in 50% glycerine and observe under microscope (Fig. 5.7).
1. Selaginella kraussiana — Haplostele:
The simplest type of stele is a protostele in which there is a solid column of vascular tissue with no pith. Xylem is exarch. It is of three types — haplostele, actinoostele, and plectostele. In haplostele there is a smooth core of xylem surrounded by phloem.
In Selaginella kraussiana the condition is distelic as there are two haplosteles. They remain suspended in an air space by cells of endodermis. Each stele has a separate pericycle.
2. Psilotum nudum — Actinostele:
In this type xylem is star-shaped with a number of radiating rays and phloem surrounds it.
3. Lycopodium clavatum — Plectostele:
Here xylem and phloem are intermingled and the two tissues occur in the form of several alternating plates.
4. Marsilea minuta — Amphiphloic siphonostele:
Siphonostele or tubular stele is characterised by the presence of a central pith. In amphiphloic siphonostele, phloem occurs on both inner and outer side of xylem.
5. Salvinia natans — Ectophloic siphonostele:
In this type of siphonostele, phloem occurs only on the outer side of xylem. In Salvinia natans the xylem ring is delicate and broken up into several segments and phloem occurs in patches.
6. Dryoptris felixmas, Helianthus annuus — Dictyostele:
In this type the leaf-gaps are fairly large and overlapping. Consequently, the stele becomes dissected into a net-like structure.
This is called a dictyostele, dissected siphonostele or eustele. The strands of vascular tissues which resemble miniature protosteles are known as meristeles.
7. Zea mays — Atactostele:
Here, the numerous vascular bundles remain scattered in the ground tissue, so that resemblance of a stele is lost. Atactos means without any order.
8. Root of Pisum sativum — Radial stele:
In this type the stele is pithless, so it is a protostele. Xylem and phloem occur along alternating in separate patches. This is called a radial stele.
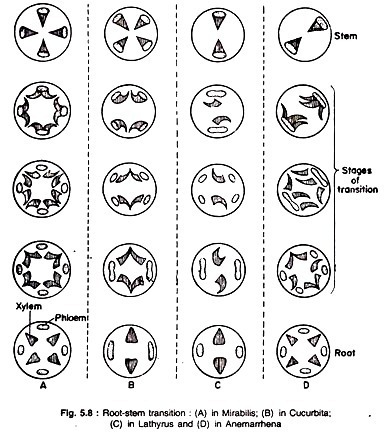
Root-Stem Transition:
The different tissues of root and stem form a continuous system. Epidermal and ground tissues are directly continuous in the two organs. But the vascular tissues of roots and stems are remarkably different as regards their arrangement: stems usually have collateral bundles with endarch xylem and roots have radial bundles with exarch xylem.
In a relatively short region of the axis, which lies at the top of the radicle or in the hypocotyl region, a change in the course of vascular tissues takes place so that root and stem vascular tissues becomes continuous. This change is called vascular transition and the region where it occurs is the transition region.
This may occur in different ways (Fig. 5.8):
Material:
Seedlings of Mirabilis, Cucurbita or Phaseolus, Lathyrus and Anemarrhena (Liliaceae). For a clear demonstration of root-stem transition serial microtome sections are necessary.
For ordinary classwork purpose, cut T.S. of the region where radicle meets plumule, i.e., the entire epicotyl-hypocotyl region, arrange the sections in a straight line on a slide in 50% glycerine, maintaining their seriality and observe under microscope. The sections can also be suitably double-stained.
Type A.:
Mirabilis:
The xylem strands of root fork radially and the two resultant halves swing laterally by 180°, one to the right and the other to the left, and join the phloem strands which remain unchanged. Thus radial bundles with exarch xylem of roots become collateral bundles with endarch xylem of stems. The number of bundles in the stem is equal to the number of phloem strands.
Type B.:
Cucurbita and Phaseolus:
In this type both xylem and phloem strands of roots become forked. The phloem strands do not rotate, but the xylem strands swing laterally as in the first type and join with the phloem strands. The number of vascular bundles in stems is twice the number of phloem strands in roots.
Type C.:
Lathyrus:
In this type, the xylem strands do not divide but swing laterally by 180° as in the previous types. The phloem strands become forked and the branches swing and ultimately join laterally and to this a strand of xylem also joins. So, the number of vascular bundles in stem is equal to the number of phloem groups present in root.
Type D.:
Anemarrhena:
In this type, half of the xylem strands fork and swing, but the other half (alternating ones) do not divide but swing by 180° as before.
The phloem strands do not divide but simply fuse laterally in pairs to which unite three strands of xylem: the middle one — an undivided xylem strand and the two lateral ones — divided strands of xylem. The number of vascular bundles in stem is equal to half the number of phloem groups in root.