Laboratory Diagnosis of Parasitic Diseases. The types are: 1. Amoebic Infections 2. Kala-Azar 3. Malaria 4. Ascariasis 5. Enterobius Vermicularis 6. Hookworms 7. Filarial Worms 8. Guinea Worm 9. Taenia Saginata 10. Taenia Solium 11. Hydatid Cysts 12. Paragonimiasis 13. Schistosoma Haematobium 14. Schistosoma Japonicum.
Parasitic Disease: Type # 1. Amoebic Infections:
Definitive diagnosis of amoebiasis is made by detection of E. histolytica in tissues or discharge from the lesions. Cultures are not employed for diagnosis of intestinal amoebiasis and serological tests are sometimes useful in extra-intestinal amoebiasis.
Acute Amoebic Dysentery:
Laboratory diagnosis:
Specimen:
Stool, blood-stained mucous, colonic scrapings from edge of ulcerated areas. Macroscopically the stool is blood and mucous mixed, copious, dark red and smells offensive, acidic and does not adhere to the container.
Specimen should be examined as soon as possible after collection, preferably within 15 minutes, because amoeboid movement of trophozoite is rapidly lost in cold surroundings. A normal saline preparation and an iodine mount of sample (2% iodine solution are made). The saline preparation is used for studying the trophozoites and iodine preparation shows morphological details of cysts.
Microscopy:
Stool:
Saline preparation:
A sample of fresh faeces containing mucous and blood is transferred on a slightly warm slide and covered by a cover glass and examined microscopically.
Observations include:
(a) Motile trophozoites throwing pseudopodia and containing red blood cells found in large number. Endoplasm is bluish or ground glass appearance and nucleus is not visible but a faint outline may be detected (Fig. 15.1).
(b) Cyst has a smooth and thin cell wall and contains round, refractile chromatoid bars. Glycogen mass is not visible (Fig. 15.2a).
(c) Red blood cells and pus cells are found in fair number.
(d) Charcot-Leyden crystals, diamond-shaped, clear and refractile structures and often present in faeces.
Iodine preparation:
It is rarely necessary for diagnosis:
(a) Trophozoite stains yellow to light brown. Nucleus is clearly visible with a central karyosome.
(b) Cyst: Cytoplasm shows a smooth and hyaline appearance. Nucleus is clearly seen and no more than 4 nuclei are present (Fig.15.2). Glycogen mass stains brown while chromatoid bars are not stained.
The morphological differences of Entamoeba histolytica and Entamoeba coli are shown in Table 15.1, Figure 15.3 and that between E. histolytica and other intestinal amoebae are shown in Table 15.2 and Figure 15.3.
Microscopical differentiation of E. histolytica from the non-pathogenic E. dispar is difficult as they are morphologically indistinguishable. The exceptions are absence of ingested RBCs in their cytoplasm. Zymodeme analysis requires culture and difficult. Antigen detection kits are now commercially available to distinguish the two strains.
Mucosal scrapings:
Mucosal scrapings obtained by sigmoidoscopy serve as an useful adjunct to conventional stool examination for ova and cyst (O and C) in cases of suspected amoebiasis.
(i) At least six samples of mucosa are to be sampled for accurate results.
(ii) Examination methods include a direct wet mount, a permanently stained smear and immune stained smears (employing either immune fluorescent or immune enzymatic staining).
Chronic Amoebiasis and Carriers:
Emphasis is made on detection of cyst of E. histolytica in faeces for establishment of diagnosis.
Identification of cyst-passers is necessary to diagnose chronic amoebiasis, detect carriers and in epidemiological survey.
Extra-intestinal Amoebiasis:
Liver, lung or brain biopsy samples are subjected to routine histology (H and E section) and Giemsa-stained touch preparation which will reveal trophozoites of E. histolytica in extra-intestinal lesions. Trophozoites can be detected in the scraping material from the wall of amoebic abscess, and rarely from aspirated anchovy sauce like pus or expectorated sputum.
Amoebic Liver Abscess:
The pus of amoebic liver abscess is distinctive red brown “anchovy-sauce”-like appearance. Since the amoebae actively multiply in the walls of abscesses, the last material aspirated is likely to contain trophozoites and may be detected by direct microscopic examination or in HE or PAS stained smears.
Serological test:
Serological tests become positive only in invasive amoebiasis. Tests for antibodies of E. histolytica in blood assist in diagnosis of extra-intestinal infections.
Serological tests include Indirect haemagglutination assay (IHA), Enzyme-linked immunosorbent assay (ELISA), Latex agglutination (LA) test, Gel diffusion precipitation (GDP), and Counter—current Immuno-electrophoresis (CIE), etc. While LA and ELISA are highly sensitive, but they often give false-positive results. Serological tests remain positive for several years even after successful treatment.
(a) Indirect haemagglutination assay (IHA):
Titre:
1: 256 to 512 – Possible extra-intestinal amoebiasis.
1:1,024 or over – Extra-intestinal amoebiasis.
(b) ELISA:
Results comparable to IHA.
(c) Cellulose acetate precipitin (CEP) test:
It has got high specificity in extra-intestinal amoebiasis.
Culture:
Cultures are not done routinely, but sometimes proved positive in cases negative by microscopy.
The following media are in use:
(i) Boeck and Drbohlav’s media of Lock’s-egg- serum medium or Lock’s-egg-albumin medium. This media has been modified by Laidlaw replacing glucose with starch. It is extensively used for isolation and maintenance of E. histolytica. Other culture media of this type include Cleverland and Sander’s medium, Nelson’s medium, etc.
(ii) Monoxenic medium: It is obtained by growing E. histolytica with Trypanosoma cruzi.
(iii) Diamond’s axenic medium is useful in the studies on pathogenicity, antigenic characterisation and drug sensitivity. It does not require presence of other microorganisms.
Growth is obtained in all these media by inoculation with a small amount of stool following incubation. Supernatant fluid is examined microscopically for presence of trophozoites of E. histolytica.
Note:
Immunity:
Immunity Infection with invasive strains of E. histolytica induce both humoral and cellular response. The immune response is greater in persons having extra-intestinal amoebiasis. Infection offers some degree of protection as evident by very low frequency of re-currency and recurrence of invasive colitis and liver abscess in endemic areas.
Parasitic Disease: Type # 2. Kala-Azar (Old World Visceral Leishmaniasis):
Visceral leishmaniasis (VL) is caused by L.d. donovani and L.d. infantum in Old World. VL caused by L. donovani was first characterised in India where it is also known as Kala-azar (meaning black sickness), Dum Dum fever or tropical splenomegaly. The two main species tend to cause similar disease, although L. infantum infects mainly young children, and has a greater tendency to cause lymph node enlargement.
Specimen:
(i) Peripheral blood.
(ii) Aspirated material of bone marrow, spleen or enlarged lymph node.
The various laboratory procedures adopted in diagnosis of kala-azar are summarised in Fig. 15.4.
A. Direct evidence:
The demonstration of Leishmania is a most important conclusive evidence of diagnosis of kala- azar. The parasite is demonstrated in amastigote form on microscopic examination on stained film of the specimen from patient and promastigote form on culture.
1. Microscopy of stained film:
Amastigote form of L. donovani is detected in Leishman or Giemsa stained smears of bone marrow or spleen and rarely from that of peripheral blood.
(a) Blood:
Occasionally amastigotes can be found in peripheral blood mononuclear leucocytes and less often in neutrophils in buffy coat smear or thick smear stained by Giemsa or Leishman stain. Blood collected in an anticoagulant (EDTA) is centrifuged in an Wintrobe tube.
The uppermost layer of white cells (buffy coat) is carefully withdrawn by a Pasteur pipette and smear made. A thick film prepared by a large drop of blood may show amastigotes in monocytes.
(b) Bone marrow aspiration biopsy:
It is the most common and useful technique for direct demonstration of the parasite in kala-azar, particularly in the early cases where spleen is not sufficient enlarged to be punctured.
(c) Splenic puncture is one of the most valuable methods for confirming the diagnosis of kala-azar.
Bone marrow puncture is a safer procedure compared to spleen puncture as there is risk of haemorrhage in splenic puncture. However, other workers consider the procedure of spleen puncture to be safe when performed by an expert and the patient’s platelet count is not less than 40,000/cumm and prothrombin time is normal. Amastigote forms of parasite are found in stained films.
Result of aspiration biopsy:
The positive result of examination of aspirates by stained smear and culture for amastigotes is:
Examination of aspirated material:
The aspirated material is examined as:
(i) Smear preparation:
At least 2 thinly-spread smears are prepared on clean slides immediately after aspiration. Only a small quantity of aspirate is necessary. Dilution of material with blood is to be avoided. The remaining aspirate is utilised for culture or placed into a tube containing EDTA anticoagulant and mixed. When the aspirate is diluted with blood, centrifuge the EDTA sample and examine the deposit as for peripheral smear.
(ii) The smears are rapidly air-dried and fixed in a few drops of absolute methanol.
(iii) Fixed smears are stained by Giemsa or Leishman stain.
(iv) When dry, add a drop of oil on smear and examine the smear microscopically under a 100 x oil-immersion objective to identify the amastigote.
Amastigote of Leishmania species (Fig. 15.5 to 15.7):
(i) Small round to oval bodies, 2-4 μm in diameter, depending on species.
(ii) Usually found in groups inside mononuclear phagocytic cells or lying free between cells.
(iii) Nucleus and rod-shaped kinetoplast in each amastigote stain dark reddish-mauve. Both structures must be observed.
(iv) Cytoplasm of the amastigote takes pale staining and is often difficult to see especially when the parasites are present in groups.
The amastigotes of different species of Leishmania are structurally similar. There are, however, variations in size between species.
LD bodies should be distinguished from Toxoplasma gondii and Histoplasma capsulatum, both of which are also intracellular organisms of monocyte- macrophage lineage of cells. The most distinguishing structure is kinetoplast of LD body which is lacking in T. gondii and H. capsulatum.
Isolation (culture):
Culturing of aspirates in visceral leishmaniasis is useful in detecting light infections. In Indian kala-azar, blood culture may establish a diagnosis without the need of spleen aspiration. Moreover, culture is also useful in checking for infection during and after treatment. The form of parasite found in culture is promastigote (Fig. 15.8).
Choice of culture medium:
Both Novy-Nicolle- McNeal (NNN) medium and Schneider’s enriched insect tissue culture medium are recommended for the in vitro culture of Leishmania. Tissue culture medium is more sensitive than NNN medium.
1. NNN medium:
Preparation of medium:
Difco blood agar base – 8 gm.
Glass distilled water – 200 ml
Defibrinated rabbit blood (10%) – 20 ml
Add the agar base in water, dissolve in a water bath and then sterilize in autoclave at 121 °C for 15 minutes. Allow the agar to cool to 45°-50°C. Add aseptically 20 ml defibrinated rabbit blood. Mix gently and dispense the medium in 5 ml amounts into 20 ml sterile screw-capped bottles (MCcartney type are preferable). Allow the medium to solidify with the bottles in a sloped position.
Inoculation:
Specimen must be collected aseptically as bacterial contamination kills Leishmanial parasites:
(a) Two bottles of culture are aseptically inoculated with 0.1 ml of specimen in each.
(b) Incubate at 24°C (±2°C) for 4 weeks.
(c) Using a sterile wire loop transfer a drop of the culture to a slide every 4 days and examine after placing a cover glass by 40 x objective. Look for motile flagellated promastigotes of leishmania (Fig. 15.8).
(d) Subcultures are made from negative cultures after 8 days into fresh medium. The primary culture is examined every 4 days for promastigotes for a further period of 3 weeks.
2. Schneider’s insect tissue culture medium:
Preparation of medium:
Schneider’s Drosophila tissue culture medium 80 ml
Foetal calf serum 20 ml
Antibiotic-antimycotic soln. 1.2 ml
All the ingredients are available commercially from “Gibcon Ltd.”. Add 20 ml inactivated calf serum (heated at 56°C x 1/2 hr) to 80 ml of Schneider’s medium and mix. Then add 1.2 ml antibiotic-antimycotic solution. Dispense the medium in 3 ml amounts in 16 x 100 m sterile tubes and stopper. Preserve the media at -20°C.
Inoculation:
Bring the media to room temperatures. About 0.1 ml specimen is inoculated into the each tube. Incubate in dark at 24°C (±2°C) for 14 days.
Examine daily a loopful of culture making a cover-slip preparation for motile promastigotes under 40 x objective.
Animal inoculation:
Animal inoculation (hamster) studies, though useful, are not employed for routine diagnosis. Golden hamsters are inoculated intranasally for cutaneous and mucocutaneous leishmaniasis and peritoneally for visceral leishmaniasis with specimen, and killed after 2 to 3 months. Parasite can be seen in smears taken from the organs (liver and spleen).
Demonstration of antigen:
Immuno-blotting (Western blot test) PCR have been developed for detection of leishmanial antigens.
B. Indirect evidence:
1. Stood examination:
The blood picture in an established case of kala-azar include:
(a) Leucopenia with neutropenia, average leucocyte count – 3,000/cumm.
(b) Erythrocyte count is also decreased.
(c) Thrombocytopenia – platelet count is reduced to about 100,000/cumm.
(d) Serum globulin level is increased.
2. Serological test:
Important serological tests comprise of a non-specific aldehyde test, specific test for detection of anti-leishmanial antibodies and leishmanial skin test.
(a) Non-specific tests:
(i) Napier’s aldehyde test (Formal-gel test):
The test depends on increase of non-specific polyclonal serum globulin in the patient’s serum. The aldehyde test becomes positive only when the disease is more than 3 months’ duration.
Test:
Take 1-2 ml of serum in a test tube, add 2 drops of concentrated formalin (40% v/v) and mix well. Allow to stand up to 20 minutes.
Interpretation:
A positive reaction is indicated by jellification of milk-white opacity like white of a hard boiled egg, usually in the course of 2-20 minutes. The reaction is called strongly positive. A milky appearance of serum without solidification may, occur in 5-20 minutes in early visceral leishmaniasis.
Aldehyde test is also found positive in infections with S. japonicum and T. brucei. It is also positive in other hypergammaglobulinaemia Multiple myeloma and Cirrhosis liver. Aldehyde test is negative in cases of cutaneous leishmaniasis.
(ii) Test with WKK antigen:
In the past decades, non-specific (non-leishmanial) antigen prepared from human tubercle bacillus by Witebsky, Klingenstein and Kuhn (hence referred to as WKK antigen) was employed for serological tests. The test is based on the fact that all mammal leishmaniae share some antigens between themselves as well as with some mycobacteria.
Complement fixation test with WKK antigen becomes positive early in disease. However, the test also becomes positive in some other conditions, e.g. tuberculosis, leprosy and tropical eosinophilia. The test is only of historic interest and is no more in use.
(b) Specific tests:
Serological test for anti-leishmanial antibodies:
In active visceral leishmaniasis, there is production of specific as well as non-specific polyclonal IgG and IgM antibodies. Specific anti-leishmanial antibodies can be detected in patient’s serum by indirect immunofluorescence test (IFAT), counter Immuno-electrophoresis (CIE) and enzyme-linked immunosorbent assay (ELISA).
3. Leishmanin skin (Montenegro) test:
Intradermal injection of 0.1 ml of killed promastigote suspension on the dorsoventral aspect of the fore arm shows negative result after 48-72 hrs. in active visceral leishmaniasis and in diffuse cutaneous leishmaniasis (DCL). Positive result is indicated by an induration of 5 mm or more.
Leishmanin test is a delayed hypersensitivity reaction, useful for epidemiological surveys of a population to find out groups at risk for infection. A combination of tissue smears (bone marrow, aspiration from spleen or lymph node), culture and animal inoculation may be required to optimize the laboratory diagnosis of leishmaniasis.
Parasitic Disease: Type # 3. Malaria:
Causative agents of malaria:
1. Plasmodium vivax, cause benign tertian malaria.
2. Plasmodium falciparum, causes malignant tertian malaria.
3. Plasmodium malariae, cause benign quartan malaria.
4. Plasmodium ovale, cause benign tertian malaria.
Malarial parasites of animals:
Several species of Plasmodium cause natural infection in animals and birds.
Parasites other than those of primates cannot infect man and that too occurs rarely:
1. Monkey malarial parasites include P. cynomolgi, P. inui, P. knowlesi, P. shortti, P. brasilianum.
2. Malarial parasites of birds are P. gallinaceum (fowl), P. relictum (passerine birds).
Laboratory diagnosis:
I. Demonstration of parasite:
A. Examination of thin and thick blood film:
Microscopic demonstration of parasites in the blood smears is the definitive method of diagnosis of malaria. Examination of both thin and thick smears or films of blood is recommended (Fig. 15.10). Thin film is useful for identifying the specific species and thick film is a concentration method in more volume of blood to detect the presence of organisms.
Specimen:
Blood films should preferably be prepared directly from capillary blood. In case of EDTA anti-coagulated blood, smears are to be made within an hour of collection of blood.
Although blood films can be taken at any time over the course of infection before starting treatment, but the best time is the midway between paroxysm of chills and fever (a few hours after the peak of the fever), when the greatest number of intracellular organisms are present. It may be necessary to take repeated films at intervals of 5 to 6 hours.
(a) Thin smear:
Wipe off the first drop of blood. The next drop of blood is touched with a clear dry glass slide, near one end. The blood is spread evenly and thinly with the edge of a spreader slide.
The film should stop before it reaches the edge of the slide. The thin, feathered end should be at least 2 cm long, and the film should occupy the central area of the slide. Thin smears are one blood cell in thickness. The film is dried in air (Fig. 15.9).
(b) Thick smear:
Usually 2 to 3 drops of capillary blood are directly placed from the finger prick to other end of the same slide as for thin smear or onto another slide. With the corner of another slide produce a square or circular patch of about 10 mm diameter.
Continue stirring for 30 sec to prevent the formation of fibrin strands that may obscure the Plasmodia after staining (Fig. 15.9). The optimum thickness of thick film is that which will just allow printed letters to be read through it.
Staining:
Both films are air dried. The thick film is dehaemoglobinised by placing the film in distilled water in a vertical position in a glass cylinder for 5 to 10 minutes and then dried in air in upright position.
Both the films are now stained with Leishman’s or Giemsa’s stain. Other stains like Wright’s or Field’s stain or JSB (Jaswant Singh and Bhattacharji) stain may also be used.
Examination:
All asexual erythrocytic stages, as well as gametocytes can be seen in the peripheral blood in P. vivax (Fig. 15.10), P. ovale and P. malariae infections, but in P. falciparum infection, only ring form and gametocytes can be seen (Fig. 15.11).
The typical text book presentation of the blood smears may not be always seen. A thin film is examined first and if parasites are found, there is no need to examine the thick film.
If parasites are found in thick film, the thin film is re-examined thoroughly to determine the species:
1. Ring form (young trophozoites) of all species appear as streaks of blue cytoplasm with detached nuclear dots but species cannot be differentiated from their appearance.
2. Thick and thin blood films should be examined carefully for presence of characteristic rings of P. falciparum (Fig. 15.11), which frequently occur in multiples within a cell, as well as in the accole position. The presence of distinctive crescentic gametocytes in RBC is diagnostic of P. falciparum.
3. Schizonts and gametocytes more or less retain their normal appearances although, they appear smaller and less regular in outline. Presence of Schuffner’s dots is also useful identifying point.
(a) Banana-shaped intra-erythrocytic gametocytes (crescents) identify P. falciparum infection.
(b) Enlarged RBC with intracellular, coarse, brick- red stippling (Schuffner’s dots) are characteristic of P. vivax infection.
(c) Schuffner’s dots in oval-shaped RBCs are characteristic of P. ovale infection.
4. Mixed infection:
Careful search in blood films should be made for mixed infections. Although, mixed infections can occur with any combination of the four species, most frequently the combination is P. falciparum and P. vivax.
Approximate quantitation of parasites:
When parasites (trophozoites, schizonts and gametocytes) are found, an approximate number of parasites per thick film field (100 x objective) is:
+ = 1-10 parasites per 100 thick film fields.
++ = 11-100 parasites per 100 thick film fields.
+++ = 1-10 parasites per thick film field.
++++ = More than 10 parasites per thick film field.
The morphology of the four species of Plasmodium that infect humans in thin films are demonstrated in Figures 15.12 to 15.15 and summarised in Table 15.3. Identification is not difficult when abundant organisms are found.
B. Quantitative buffy coat smear:
Parasites may be concentrated by micro-haematocrit centrifugation using glass capillary tube and closely fitting plastic insert (QBC malaria blood tubes, Becton Dickinson, Sparks, Md, USA). The QBC (quantitative buffy coat) tube is a specially prepared glass haematocrit tube, pre-coated internally with acridine orange stain and potassium oxalate.
A volume of 56-65 micro-litres of blood collected from finger, ear or heel puncture and centrifuged at 12,000 rpm for 5 minutes. RBCs containing malarial parasites are less dense than normal red cells and concentrate just below the leucocytes at the top of the erythrocytic column.
The parasite contains DNA but the mature RBCs do not contain DNA and RNA. Parasitic DNA is detected by acridine orange stain and appear as bright specks of light among the non-fluorescing erythrocytes. When the QBC malaria blood tubes are rotated under a special type of lens (paralens), almost all the plasmodia in the blood sample can be visualised.
C. Micro-concentration technique:
Blood is collected in micro-haematocrit tube and centrifuged at high speed. The sediment is mixed with normal serum and smear prepared. Although, the positivity rate is increased by this technique, but there is change in morphology of the parasite.
II. Immunodiagnosis:
Serology cannot match the sensitivity of microscopic detection of malarial parasite, but may assist diagnosis of light infection. It has got limited value in clinical diagnosis because they will not differentiate between an active and a past infection. These antibody test including indirect fluorescent antibody (IFA) test and indirect haemagglutination assay (IHA), ELISA and RIA are useful for epidemiological purposes.
III. Newer approaches to diagnosis:
A. Rapid and simple stick test (Para-Sight F test, Becton Dickinson, Europe):
This test is based on detection of a monoclonal antibody against P. falciparum histidine rich protein 2 antigen. This test is based on antigen capture approach and has been incorporated in a dipstick format.
Antibodies specific for P. falciparum HRP 2 are immobilised in the dipstick (test strip) when dipped in blood. A solid pink line indicates positive test. This test is very useful in treatment and diagnosis of drug-resistant P. falciparum.
B. Use of fluorescent dye:
Benzo-thio-carboxy-purine (fluorescent dye) intensely stains nucleic acid of malarial parasites after penetrating RBCs. The dye does not stain nuclei of WBCs. This is useful in field laboratory for mass screening because of rapid staining and evaluation but it requires a fluorescent microscope.
C. Detection of parasitic antigen:
Detection of malarial antigens by RIA, DNA probes and PCR are under evaluation.
Parasitic Disease: Type # 4. Ascariasis:
A. Direct evidences:
1. Demonstration of adult worm:
This is sometimes possible in the following situations:
(a) When adult worm is spontaneously passed in stool or vomitus.
(b) Administration of antihelminthics may result in expulsion of worm and its detection.
2. Detection of egg:
Ascaris egg can be detected in stool by direct wet mount and also by concentration method.
(a) Direct wet mount:
Diagnosis is usually made microscopically by finding of eggs in a direct saline preparation of faeces. Since many eggs are produced, concentration techniques are rarely required. A single female A. lumbricoides may account for 3 eggs per mg of faeces.
Usually both fertilised and un-fertilised eggs are found (Table 15.4). Fertilised eggs may sometimes appear decorticated. Rarely semi-decorticated un-fertilised egg is encountered (Table 15.4). When a patient harbours only male parasite(s), eggs are not found in the stool.
(b) Concentration technique:
Occasionally, when the worm infestation is light, concentration technique may be necessary. Un-fertilised eggs are not detectable by salt floatation. Sedimentation technique is more sensitive than floatation method.
(c) Duodenal aspirate of an infected patient collected by duodenal intubation also shows eggs.
3. Demonstration of larva in sputum:
Ascaris larvae may be detected in sputum in Loeffler’s syndrome along with eosinophil’s and Charcot- Leyden crystals.
B. Indirect evidences:
1. Blood examination:
Eosinophil count may be increased at the early stage of infection.
2. Skin test:
Skin test with A. lumbricoides antigen gives a positive result, but is unreliable and not used for diagnosis.
3. Serological test:
Serological tests are of particular use in Ascaris pneumonitis. The available tests include ELISA, IHA and micro-precipitation on larvae. Although these tests are useful in serosurvey, but is not useful in routine diagnosis.
Parasitic Disease: Type # 5. Enterobius Vermicularis:
1. Cellophane tape test:
Diagnosis is made by finding E. vermicularis eggs in morning samples collected from perianal skin by applying the adhesive surface of cellophane tape or by NIH (devised by National Institute of Health, USA) swabs (Fig. 15.16).
The tape is mounted in water or 0.1 M sodium hydroxide on a slide with the adhesive side down, covered with a cover slip and examined under a microscope. Other methods (e.g. the use of anal swabs) have also been used effectively to demonstrate enterobius infections. The ova (about 55 x 25 μm in size) are oval, flattened on one side and usually contain a larva.
(a) Specimens are to be collected prior to bathing or going to toilet.
(b) Four to six consecutive negative finding of tape preparations are required to rule out pinworm infection.
2. Stool samples:
(a) Eggs may also be found in faeces in patients with heavy worm burdens.
(b) Adult female worms may be found in faeces.
Parasitic Disease: Type # 6. Hookworms:
Species:
Group I: True human parasites (Anthrophilic):
Group II: True human parasites (Anthrophilic):
1. Ancylostoma braziliense found in dogs and cats in Brazil.
2. A. caninum — found in dogs and cats in most tropical areas.
3. A. ceylanicum (According to some it is nothing but A. braziliense) found in civet cat in Sri Lanka.
4. Uncinaria stenocephala found in dogs, cats and foxes in Europe and North America.
Ancylostomiasis, caused by these two principal human hookworms, is one of the main causes of anaemia in tropics. Other species of the genus Ancylostoma also may produce human infections in various parts of the world.
Necator americanus is found in USA as well as other parts of the world, but A. duodenale does not occur in the USA, although its geographical distribution elsewhere in tropical and subtropical Asia overlaps that of Necator.
All hookworms have common morphologic characteristics with some minor difference. Infection with N. americanus is more common throughout India, South-East Asia, Africa and S. America. A. duodenale is found in Middle-East, Mediterranean and in Punjab and UP in India.
The zoophilic (animal) parasites rarely or never mature in man and cause creeping eruption or cutaneous larva migrans.
Laboratory diagnosis:
The diagnosis of hookworm infection is made by demonstration of eggs in the faeces by direct microscopy or by concentration methods. The species identification can be made by distinguishing adult worms and the mature filariform larvae (Table 15.5, Fig. 15.17).
I. Direct evidence:
1. Examination of stool:
Demonstration of characteristic eggs in direct wet mount of faeces establishes diagnosis. Eggs of Ancylostoma and Necator are morphologically indistinguishable. However, if direct smear is negative, a concentration smear should be made.
Routine examination:
(i) Microscopic examination of a direct faecal smear (saline mount) is made. With counts of fewer than 5 eggs per cover slip preparation indicate light infections unlikely to produce anaemia. Counts above 25 eggs per cover- slip indicate heavy infection.
(ii) When there has been a prolonged delay in examination of the stool samples (usually more than a day), larvae may develop and hatch.
The first-stage rhabditiform larvae) of hookworm has to be differentiated from those of Strongyloides stercoralis, the stage typically passed in stool of human strongyloidiasis. The rhabditiform larva of hookworm has a long, narrow buccal chamber and an inconspicuous genital operculum (Fig. 15.18).
(iii) The species differentiation of hookworm can be made by studying adult worms or culturing larval stages to the infective filariform stage with subsequent differentiation of larval forms (Figs. 15.17 and 15.19).
Concentration method:
Concentration methods are useful in detection of light infection and to assess the worm load.
The following techniques are usually followed:
A. Formal ether sedimentation technique:
About a gram of faeces is emulsified in 7-10% formol saline (fixative) and left for 10 minutes. It is then strained by an wire gauge (40 meshes to an inch) in a centrifuge tube. Add 3 ml ether to the filtrate and shake vigorously for one minute and then centrifuge at 2,000 rpm for 2 minutes. Allow to settle.
Supernatant fluid with debris is decanted leaving 1-2 drops. Cover-slip preparation from the deposit is examined for ova and cyst. In this technique cysts, eggs and larvae are fixed and sedimented. Count the number of eggs which give the approximate number of ova per gram of faeces.
B. Salt floatation technique:
Common salt or zinc sulphate are used for concentration of ova, cysts and larvae.
(a) Saturated sodium chloride technique:
The faecal sample is dissolved in a solution of higher density so that eggs float on surface.
Eggs float after 20-30 minutes and begins to sink after 60 minutes:
(i) One quarter of a 20-25 ml test tube is filled with saturated solution of sodium chloride.
(ii) About one gram of faeces is emulsified in the solution using a glass rod or stick and then 12-15 ml salt solution is added.
(iii) The tube is kept in a completely vertical position on a rack. Any coarse matter may be removed.
(iv) Using a Pasteur pipette, further solution is added to ensure that the tube is filled to the brim.
(v) A cover glass is carefully laid on the top of the tube, so that the cover slip is in contact with the fluid.
(vi) The preparation is allowed to stand for 30- 35 minutes undisturbed for the cysts and eggs to float.
(vii) The cover glass is lifted carefully from the tube by a straight pull upwards and placed on slide with face downwards.
(viii) Examine under low power (10 x objective) and count the number of ova which gives an approximate idea of the number of ova per gram of faeces.
Application:
Common salt floatation method is useful for concentrating Ascaris eggs or hookworm eggs in field surveys.
(b) Zinc sulphate floatation method:
This technique is similar to that of salt floatation method except that 33% zinc sulphate solution is used in place of saturated sodium chloride. Slide is prepared in the same way.
During microscopy, the unstained preparation is first examined, and then a drop of iodine is placed under the cover glass and viewed under high power (40 x). Cysts of Giardia lamblia and E. histolytica and some other ova float in the method. The cysts appear distinct in iodine preparation.
Table 15.6 shows the comparative result of the three concentration tests:
2. Study of duodenal contents:
Aspirate obtained from duodenum by duodenal intubation (Ryle’s tube) may sometimes contain eggs or adult hookworms.
3. Culture:
Stool culture may be done using Harada-Mori filter paper strip technique or charcoal culture method. It is necessary to distinguish the filariform larvae of A. duodenale and N. americanus (Fig. 15.17) on epidemiological ground.
II. Indirect evidences:
1. Occult blood (benzidine) test of stool is positive.
2. Anaemia — microcytic hypochromic.
3. Blood picture — Hb, PCV, MCV, MCHC all decreased.
4. Eosinophilia in blood.
5. Charcot-Leyden crystals are found in stool.
Parasitic Disease: Type # 7. Filarial Worms:
Filarioidea (Filarial worms):
Based on normal habitats of adult worms, filarial infections can be divided into four groups which include:
A. Lymphatic filariasis:
Wuchereria bancrofti, Brugia malayi, Brugia timori.
B. Subcutaneous filariasis:
+ Loa loa
Onchocerca volvulus
Mansonella streptocerca
C. Serous cavity filariasis:
Mansonella ozzardi (mesentery)
Mansonella perstans
D. Animal tissue nematodes:
Dirofilaria:
Dirofilaria immitis (dog heartworm)
Dirofilaria tenuis (raccoon parasite)
Dirofilaria repens (dog parasite)
+ Loa loa and some animal filaria (e.g. Dirofilaria conjunctivae) also infect conjunctivae.
These animal parasites sometimes infect man and cause pulmonary dirofilariasis and erythematous subcutaneous nodules.
Eight species of filarial worms infect humans that cause filariasis, but traditionally the term filariasis refers to lymphatic filariasis attributable to W. bancrofti, Brugia malayi and Brugia timori. They can be differentiated on the basis of their location in the body and characteristics of microfilaria (Fig. 15.21).
Laboratory diagnosis:
Specimens:
(i) Microfilaria begins to appear in blood a year or more after an infection. Diagnosis is established by detection of microfilaria of W. bancrofti in blood.
(ii) Occasionally, microfilaria can be found in chylous urine or hydrocele fluid.
(iii) Sections of adult worms are sometimes detected in biopsied lymph node.
The procedures adopted for laboratory diagnosis are shown in Fig. 15.20.
A. Direct evidences:
Microscopy:
Definitive diagnosis is made by detection of microfilariae in a thick blood smear and chylous fluid.
Examination of blood:
It should be remembered that most microfilariae of W. bancrofti and B. malayi typically exhibit nocturnal periodicity.
Collection time:
1. Blood:
About 5-6 ml venous blood collected in an anticoagulant (Pot. oxalate or Sodium citrate) is centrifuged and the deposit is examined by:
(a) Thick smear technique:
Smear made from two drops of deposit is stained by Leishman/Giemsa stain and examined microscopically.
(b) Wet-slide preparation:
Two drops of deposit is mixed with equal volume of water (to lyse the red cells) on a slide. The preparation is covered with a cover slip and examined microscopically using low power (10 x objective). Larger volumes of blood increase the diagnostic sensitivity. Membrane filtration of microfilariae from 10 ml oxalated or citrated blood is better than the concentrated sediment prepared by centrifugation.
W. bancrofti, B. malayi and Loa loa demonstrate a sheath on their microfilaria (Fig. 15.21) and is the first step in the identification of specific types of filariasis. Further differentiation is based on the study of the head and tail structure of microfilariae (Fig. 15.22). W. bancrofti does not have nuclei in the tip of the tail, while B. malayi has two prominent nuclei at the tip of the tail.
An exact species identification is not critical from treatment point of view because treatment of all the filarial infections, except Onchocerca volvulus, is identical.
DEC provocation test:
A small single dose of diethyl carbamazine (2 mg/kg BW) makes microfilaria to appear in peripheral blood even during day time. After 30-50 min of administration of a 100 mg DEC tablet in an adult, blood sample is collected for detection of microfilaria. This is useful in mass screening.
2. Examination of chylous fluid:
Chylous fluid is creamy white and opalescent. Chyle consists of lymph and particles of digested fat (ether soluble). When blood is also present, the specimen appears pinkish-white. About 10-20 ml of first urine passed by the patient in the morning is collected.
Procedure:
Urine is centrifuged at 2,000 rpm for 10-15 minutes. The supernatant is thrown out and the deposit is mixed with equal volume of water and centrifuged again. The supernatant is discarded and wet preparation from deposit is placed on slide, covered by cover slip, examined for the presence of microfilariae. The deposit may be smeared on slide and stained by Leishman or Giemsa stain.
3. Examination of biopsy specimens:
Adult filarial worms can sometimes be found in sections of biopsied lymph nodes (Fig. 15.23) as incidental finding but this is not done for routine diagnosis.
4. Detection of circulating antigen:
With the development of monoclonal antibodies, detection of circulating filarial antigen has been done in reference laboratory. The result is promising but the test is not widely available as a diagnostic test.
B. Indirect evidences:
1. Immunological tests:
Specific antibodies against filarial antigen can be detected by serological tests. A hypersensitivity reaction to filarial antigen is often observed.
(a) Detection of serum antibody:
Using Brugia pahangi antigen, fluorescent antibody tests (FAT) and ELISA can detect over 95% of active cases and 70% of established elephantiasis. Cross-reactions are observed in 15% cases of strongyloides and 5% of other intestinal nematodes.
Since microfilaria are often difficult to find in blood in bancroftian filariasis, the antibody detection tests are giving promising results. The test becomes negative 1-2 years after cure.
(b) IgG subclasses antibodies against B malayi:
There is significantly higher level of filarial IgG4 antibodies in blood of microfilaraemic carriers, acute cases and in occult filariasis suggesting presence of active filarial infection in these patients (Bhunia et al, 2003). Filarial antigen detection shows significantly high level of antigen in this group.
(c) Skin test:
Intradermal skin tests using filarial antigen (extracts of microfilariae, adult worms and third stage larvae of B. malayi or of the dog filaria Dirofilaria immitis), show immediate type hypersensitivity which persists for life. The diagnostic value of the test is limited due to high false-positivity.
2. Blood count:
Eosinophilia (5-15%) is a common finding.
Parasitic Disease: Type # 8. Guinea Worm:
Causative agent:
The adult female of Dracunculus medinensis (also called guinea worm) resides in the subcutaneous tissue, especially of legs, arms and back. At the site of localisation, the worm sets up an inflammatory reaction producing a papule and then blister.
Laboratory diagnosis:
1. Detection of adult worm:
Diagnosis is made by observing the typical ulcer and the female worm at the surface of the skin.
2. Detection of embryos:
The affected (ulcer) is flooded with water that induces the release of embryos which can be examined under the microscope.
3. Intradermal test with dracunculus antigen leads to a wheal type reaction after 24 hours.
4. X-ray examination may show coiled structure or linear density (up to 25 cm) or calcified worm in deeper tissues.
Parasitic Disease: Type # 9. Taenia Saginata:
Systemic classification of cestodes:
Systemic classification of cestodes has been described in Table 15.7.
Laboratory diagnosis:
Direct evidences:
Laboratory confirmation of diagnosis of T. saginata infection is made by demonstration of gravid segments and eggs passed in faeces.
Microscopic examination of stool:
Eggs with characteristic thin shell and six hooked oncosphere, are diagnostic. Since eggs of T. saginata and T. solium are indistinguishable, the species diagnosis is to be made by examining the gravid segment (Table 15.8). Moreover, eggs are not frequently found in faeces because of absence of uterine openings.
T. saginata segment shows:
(a) 15-30 lateral branches of uterus.
(b) No accessory lobe in the ovary.
(c) Presence of vaginal sphincter muscle.
(d) Typical isoenzyme patterns.
Microscopic examination of segment:
Species identification is made by examining gravid segment. The gravid segment pressed between two slides and is examined by a hand lens that will show 15-20 lateral branches of uterus in each segment.
B. Serology:
Serodiagnosis by IHA, ELISA and IFA have been used for detection of T. saginata and T. solium coproantigens which appear to be very promising.
Diagnosis in animals:
ELISA and CIEP using oncosphere as antigen have been used with some success. In slaughtered cattle, cysticercus bovis can be seen by naked eye.
Parasitic Disease: Type # 10. Taenia Solium:
Morphology:
Adult worm:
Adult worm lives in the small intestine (upper jejunum) of man and it is 2-3 metres in length and life span up to 25 yrs. Commonly a single worm is present, but several worms up to 25 or more may sometimes be found in a patient.
Scolex (head):
Head measures 1 mm in diameter (pin-head size) and globular, possesses a rostellum armed with double rows of hooklets and 4 circular suckers (Figs. 15.24 and 15.25).
Neck:
Short, 5-10 mm long.
Proglottids:
Number 800-900, gravid segment measures 12 mm x 6 mm, being twice as long as wide. The proglottids of T. solium resemble those of T. saginata in general. Gravid uterus consists of a median longitudinal stem with 5-10 compound lateral branches on each side (Figs. 15.26 and 15.27).
In mature proglottids, the ovary has 2 lobes and an accessory lobe (in T. saginata, accessory lobe lacking), and a vaginal sphincter muscle is lacking (present in T. saginata).
Proglottids are not very active. The gravid segments are not passed singly, but pass out passively as short chains.
The differential characteristics of gravid adult T. solium and T. saginata are shown in Table 15.6.
Laboratory diagnosis:
1. Intestinal taeniasis:
Laboratory confirmation of diagnosis is made by:
(a) Identifying gravid segments passed in faeces.
(b) Detection of eggs in faeces.
2. Human cysticercosis:
(a) Calcified cyst in muscles can be recognised radio logically and by biopsy.
(b) CT scans or MRI techniques may reveal the presence of cysticerci in the brain.
(c) Fine needle aspiration (FNA) cytology is useful and cost-effective since it eliminates the biopsy.
(d) Serologic tests is helpful in some patients but cross-reactivity is observed between cysticercosis and hydatid infection.
(e) Immunoblot and immune electro-transfer blot (EITB) using blood in antibody detection in neuro- cysticercosis are useful tests.
Eosinophilia usually occurs in the early stage of cysticercosis. An indirect haemagglutination test using antigen from cysticercus of pig has been reported to give positive result.
Parasitic Disease: Type # 11. Hydatid Cysts:
Species:
A number of cestodes (tapeworms) live as adults in the small intestine of man and animal and larval stages of some of them often occur in human tissues, e.g. cysticercosis caused by T. solium and T. saginata; sparganosis caused sometimes by D. latum.
In addition to intestinal cestodes, a number of tissue cestodes occur in larval stages in human tissue. The most significant diseases produced by larval stages of tissue cestodes include several forms of hydatid disease (Echinococcus species), coenurosis (Multiceps species) and sparganosis (Spirometra species).
The genus Echinococcus has four species which include:
(1) E. granulosus — causes hydatid cyst, the commonest echinococcal infection.
(2) E. multilocularis — causes alveolar or multilocular disease.
3. E. oligarthus — causes polycystic hydatid disease.
4. E. vogeli — causes polycystic disease.
There is clear evidence of strain variation in E. granulosus, but for other species, clear-cut evidence of strain variation does not exist.
Diagnosis:
Diagnosis of hydatid cyst is difficult and depends on clinical, radiographic, aspiration and serological findings.
1. Radiographic findings:
Radiologic examination (plain X-rays permit the detection of cyst only), ultrasound, CT scan and angiography may provide the first evidence of a cyst’s presence.
2. Exploratory cyst puncture:
Aspiration of cyst contents may reveal the presence of proto-scolices (hydatid sand); however, it is contraindicated because of accidental spilling of the contents may cause anaphylaxis and secondary spread of hydatid infection.
Hydatid sand is not always present. Moreover, daughter cysts and/or scolices may disintegrate in old cyst. So only the hooklets are left. This may cause diagnostic problem.
3. Serologic tests:
Antigen: Specific antigens (8-and 16-kDa antigens) are present in the hydatid fluid. Antibodies to the antigen appear in blood after infection. The source of antigen include sterile hydatid fluid obtained from sheep or man, cyst wall or proto-scolices.
Tests:
(a) Antibody detection:
ELISA, indirect immunofluorescence test (IFT), latex agglutination (LA) test, and indirect haemagglutination assays (IHA) are done using hydatid antigen to detect antibodies in serum. Precipitin and CFT tests with hydatid antigen are also found to be positive. Diagnostic sensitivity varies from 60-90%.
Hydatid antigen dot immunoassay:
It is a newer method of diagnosis of hydatid cyst in which serum sample is incubated with a textile colloidal dye and a nitrocellulose stick to which the hydatid antigen has been bound. The presence of E. granulosus specific antibodies leads to dying of stick reactive area, and a colour spot develops. This technique shows good correlation with other available methods.
(b) Antigen detection:
Specific antigen in sera and in CSF (in cerebral hydatid cyst) can be screened by double diffusion and counter Immuno-electrophoresis technique.
4. Casoni test:
Until recently, the Casoni test was the only means of diagnosis of hydatid disease and almost abandoned now. This is an immediate hypersensitivity (Type I) skin test introduced by Casoni in 1911.
Antigen:
Hydatid fluid collected from animal or human cysts and is sterilised by Seitz or membrane filtration.
Test:
Sterilised fresh hydatid fluid (0.2 ml) is injected intradermally on one arm and equal volume of saline as control on the other arm.
Result:
In a positive reaction, a large wheal (about 5 cm in diameter) with multiple pseudopodia-like projections appears within half an hour. The wheal fades away in an hour.
Remarks:
(i) False-positive and false-negative cases may occur. Non-specificity has been the major limitation of Casoni test. False-positive reactions have been observed with other parasitic and non-parasitic diseases.
(ii) Diagnostic sensitivity is limited in patients having intact or hyaline cysts.
(iii) The antigen may sensitize the patient leading to antibody production and anaphylactic reaction. The test is, therefore, rarely used for diagnostic purpose.
Parasitic Disease: Type # 12. Paragonimiasis:
Causative agents:
Paragonimiasis is a chronic lung infection of lungs of men and carnivores. Important species are shown in Table 15.9. P. westermani, commonly known as “lung fluke” mostly found in human infection causing an important disease.
Diagnosis:
Clinical diagnosis of paragonimiasis is suspected in an individual with chronic cough, vague chest pain and haemoptysis who have resided in an endemic area and is in the habit of eating raw or undercooked crab or cray fish.
Chest X-ray films often show infiltrates, nodular cysts and pleural effusion. Since the symptoms closely simulate those of pulmonary tuberculosis, diagnosis should be established by detection of eggs and antibodies.
Specimens:
Paragonimus eggs can be detected in sputum and faeces and concomitant examinations of both should be done. In many cases, eggs are intermittently passed in sputum and faeces. Eggs are occasionally found in aspirated pleural fluid.
A. Demonstration of eggs in sputum:
Sputum often contains blood, mucus and rusty brown particles in which eggs can be seen.
(a) Physical examination:
Watery, mucoid or mucopurulent; or red or jelly-like due to haemorrhage.
(b) Microscopy:
(i) Direct microscopy:
Carefully examine any blood tinged flecks for eggs; the egg clusters have been described as looking like iron-filings. Repeated sputum examination, at least for seven consecutive days should be examined. A saline wet mount is preferred.
When eggs cannot be found in direct examination, sample should be concentrated. The eggs possess prominent opercula, the shell is distinctly thickened at abopercular end of the shell, and they are un-embryonated when passed in faeces.
(ii) Concentration Method:
Mix equal volumes of sputum and 3 to 4% sodium hydroxide in a centrifuge tube, leave for 10-15 minutes (to dissolve the mucus) and then centrifuge at 2,000 rpm for 5 minutes. Examine the sediment microscopically.
B. Detection of eggs in Faeces:
(a) Routine microscopy is often negative in mild infection because of scanty eggs, hence concentration technique is preferable.
(b) Concentration of ova in faeces by formal ether concentration method is preferable (Fig. 15.28).
C. Serological tests:
Parasitic-specific IgG and IgE anti-paragonimus antibodies in patient’s serum detected by ELISA using adult excretory-secretory antigens is very sensitive. Dot ELISA has also been used to detect parasitic antigen in human sera.
D. Eosinophilia:
Eosinophilia is almost a constant finding.
Parasitic Disease: Type # 13. Schistosoma Haematobium:
Species:
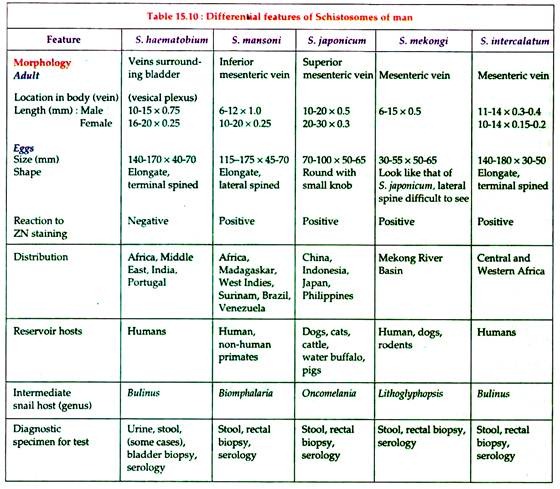
Schistosoma species with their differential characteristics are shown in Table 15.10:
Diagnosis:
A. Direct evidences:
(a) Microscopy:
Definitive diagnosis is made by detection of eggs.
1. Examination of centrifuged deposit of urine shows eggs with characteristic terminal spine. Occasionally hatched miracidia of S. haematobium may be found. Eggs may be also found in seminal fluid. They may also be found in faeces. In moderate to heavy infections, the urine is usually reddish in colour and cloudy due to presence of blood.
Since eggs are more abundant in the blood and pus passed at the end of micturition, patient should be instructed to include in the specimen the last few drops of urine passed. Freshly passed urine or urine preserved with 10% formalin should be examined for eggs. Miracidia may be found if the urine is left to stand without preservative for a few hours before examination.
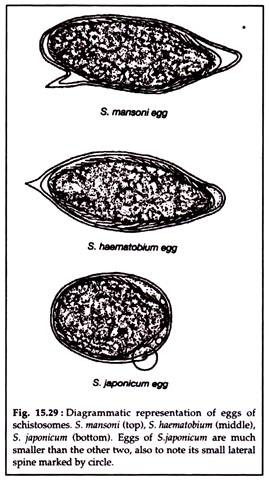
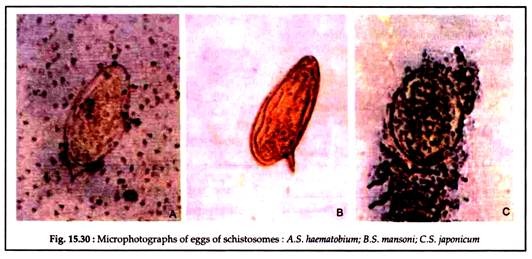
In light infections or for making total egg count, twenty-four hours sample of urine is collected and to which 10% formal saline (0.5 ml per 100 ml urine is added). The urine is either filtered by membrane filter or centrifuged and the deposit is used for microscopy (Fig. 15.29).
2. Biopsy:
Eggs may also be found in the wall of the rectum as well as in the bladder wall. Detection of eggs in rectal or bladder mucosal biopsy may be done when infection is light. The biopsied tissue may be digested with potash solution (4% NaOH/KOH solution) and later examined for eggs.
(b) Serological tests:
Serological tests may be helpful when no eggs are found (in case adult worms lie in ectopic sites, e.g. CNS, spinal vessels or orbital blood vessels). ELISA, latex agglutination, complement fixation test to detect antibodies in serum are done at some places. Animal schistosomes are used as antigen.
Plasma card test is now available for mass screening. A drop of blood from finger of a patient is taken on the plastic card to which a drop of antigen solution (prepared from adult worm or cercariae) containing charcoal powder is added. The slide is rotated for 2 minutes. Positive test shows precipitation.
B. Indirect evidences:
1. Haematuria and proteinuria, caused by eggs penetrating the wall of the bladder, is a characteristic feature of S. haematobium infection. The use of reagent strips for detecting haematuria and proteinuria in areas of endemic infection has proven as useful screening test.
2. Blood exam:
Eosinophilia is a common finding (Fig. 15.30).
Parasitic Disease: Type # 14. Schistosoma Japonicum:
Laboratory Diagnosis:
Laboratory confirmation of S. japonicum infection is similar as in S. mansoni.
1. Detection of eggs:
(a) Finding S. japonicum eggs in faeces.
(b) Rectal biopsy is an important diagnostic test when eggs cannot be found in faeces.
2. Serological tests:
ELISA, LA, CFT.
3. Other findings:
(a) Mucus and blood are often present in stool.
(b) Eosinophilia is present.