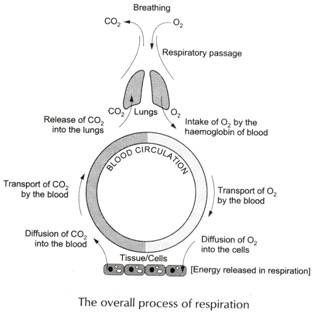
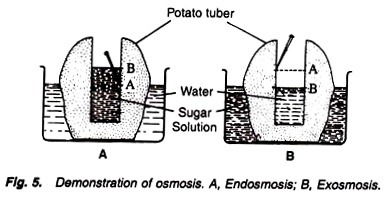
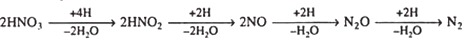Proteins are composed of one or more chains of amino acids called polypeptides.
The neighboring amino acids in the chain are linked together by peptide bonds; these bonds are formed, in effect, by the elimination of one molecule of water from the two amino acids linked by the bond, such that the a-carboxyl carbon atom of one amino acid is linked to the a-amino nitrogen atom of the neighboring amino acid (Fig. 4-4).
This type of reaction is known as a condensation reaction, or, in this particular case, a dehydration synthesis.
 The amino and carboxyl groups that are not directly bonded to the alpha carbon atom (such as those that occur in the side chains of lysine, glutamic acid, and aspartic acid) do not form covalent bonds with neighboring amino acids.
The amino and carboxyl groups that are not directly bonded to the alpha carbon atom (such as those that occur in the side chains of lysine, glutamic acid, and aspartic acid) do not form covalent bonds with neighboring amino acids.
The two atoms that are involved in the peptide linkage and the four adjacent atoms lie in the same plane in space and form what is called a planar amide group (Fig. 4-5). Primarily through the pioneering studies of L. Pauling and R. B. Corey, the specific interatomic distances and bond angles of the planar amide group are known; these are shown in Figure 4-6.
In 1954, Pauling received a Nobel Prize for his work on protein structure. In the planar amide group, the C—N bond (i.e., the peptide bond) and the C—O bond assume a character somewhere between that of a double bond and a single bond. This prevents rotation about either bond or results in the planarity of the group.
It should be noted that a planar amide group includes parts of two neighboring amino acids, and the alpha carbon atoms at each end of a planar amide group are also included at the ends of neighboring planar amide groups. The bond angle between planar amide groups is 111o; this is the angle formed between the C—C bond of one planar amide group and the C—N bond of the next group. Note that this is an intra-amino acid bond angle! Figure 4-7 shows two successive planar amide groups arranged in such a manner that they are coplanar (i.e., both planar amide groups lie in the same plane).
The alpha carbon atoms are identified simply by a. The important 111° angle formed between the C—C bond of one group (the upper group) and the C—N bond of the other (the lower group) is specifically identified. Clockwise rotation (when viewed from the position of the alpha carbon atom) of the upper planar amide group about the C—C bond sweeps out the angle called ψ(psi).
Clockwise rotation (again when viewed from the alpha carbon atom) of the lower planar amide group about the C—N bond sweeps out the angle called ψ (psi). It should be noted that the 1110 angle between the two planar amide groups remains fixed regardless of the values of ψ and ф.
The flexibility of a chain of amino acids (i.e., a polypeptide) results from the rotations of successive planar amide groups about these bonds yielding the various values of ψ and ф and does not result from the linkages between consecutive amino acids. This point cannot be overemphasized if the conformations that can be assumed by polypeptides are to be properly understood. Figure 4-8 is a stereoscopic view showing two successive planar amide groups that are not coplanar.
The total length of two (or more) planar amide groups is maximized when ψ and ф are zero. Chain length is reduced as the ψ and ф angles are altered between 0° and 360°. If these angles are kept constant within a stretch of polypeptide, the series of planar amide groups naturally takes on the shape of a helix. In theory, all kinds of helices can be formed using various values for ψ and ф.
However, in actuality, most combinations of ψ and ф are not possible, because they would result in the superimposition of atoms in space. Much of the experimental data that have been accumulated to date indicate that most polypeptides contain many regions of helical coiling and few regions of complete extension. The relative lengths of helical and extended segments of a polypeptide are determined by the specific sequence of amino acids present in the polypeptide.