Read this article to learn about Mono Saccharides: Pyranoses and Furanoses !
The most commonly occurring mono-saccharides are either aldoses or ketoses and contain three to six carbon atoms forming an un-branched chain.
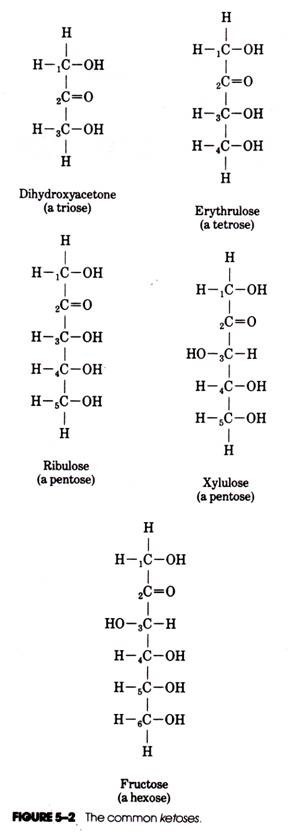
Those sugars containing three carbons are trioses, those with four carbons tetroses, those with five carbons pentoses, and those with six carbons hexoses (Figs. 5-1 and 5-2). 
In the aldoses, the number 1 carbon (called the “ultimate” carbon) forms the double bond with oxygen, whereas in the ketoses, it is usually the “penultimate” carbon.
The middle carbon atom (i.e., carbon number 2) of glyceraldehyde is asymmetric-, that is, it has four chemically different groups bonded to it, and it therefore has two optical isomers. The two isomers of glyceraldehyde are shown in Figure 5-3 and are identified as the D and L forms.
All the aldoses may be considered to be derivatives of glyceraldehyde, whereas the corresponding ketoses are derivatives of dihydroxyacetone. D or L notation is determined by similarity of the second carbon atom to glyceraldehyde and is independent of whether the sugars are dextrorotatory or levorotatory.
The most common aldoses and ketoses found in nature (e.g., those in Figs. 5-1 and 5-2) are the d forms. The number of possible isomers of a monosaccharide is determined by the number (n) of asymmetric carbon atoms present in the chain. Glucose (n = 4) has 24 or 16 possible isomers. In spite of the variety of possible monosaccharide isomers, only a few different forms occur naturally in any abundance. For example, there are only three naturally occurring isomers of glucose; these are D-glucose, D-mannose, and D-galactose (see Fig. 5-1).
Pyranoses and Furanoses:
Some of the mono saccharides can occur in either of two structural configurations: open-chain and cyclic (or ringed). Cyclic forms that contain a six-member ring are called pyranoses and those that contain a five-member ring are called furanoses. Both the open-chain and cyclic configurations are in reversible equilibrium with one another in aqueous solution, although the cyclic forms are much more prevalent.
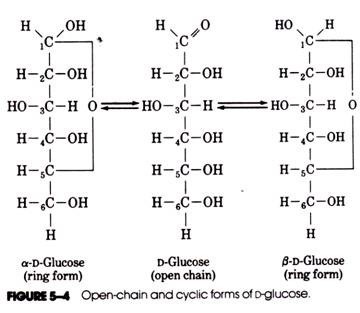
As an example, the formation of the cyclic structures of d-glucose is shown in Figure 5-4. The aldehyde group of carbon atom number 1 reacts with the hydroxyl group of carbon number 5 to produce a six-member ring containing oxygen. The formation of the ring form of glucose makes carbon number 1 asymmetric and thereby increases the number of possible isomers. The two ring forms of D-glucose are called the α and β anomers. In aqueous solutions of glucose, the β anomer accounts for about 63% of the molecules present, the α form 36%, and the open-chain form about 1%.
The three-dimensional arrangement of the constituent atoms of the ring structure of mono saccharides is more readily visualized when the structure is depicted in the Haworth projection (after W. H. Haworth) (Fig. 5-5). In this projection, the members of the ring are arranged in a plane that is perpendicular to the plane of the page.
Groups to the right of the carbon skeleton in the open-chain configuration are depicted below the plane of the ring and those to the left of the carbon skeleton extend above the plane of the ring. Certain bonds between members of the ring are deliberately shaded more heavily to assist the viewer’s perception of the three-dimensional perspective.
The Haworth projection of glucose (and other pyranoses) in which the members of the ring are all depicted as lying in the same plane is not entirely accurate. Though carbon atoms 2, 3, and 5 and the oxygen atom lie in the same plane (to form the corners of a square), carbon atoms 1 and 4 lie either slightly above or below the plane.
This gives rise to the so- called boat and chair forms of glucose (Fig. 5-6), of which the chair form is energetically favored. Other cyclic aldohexoses also exhibit boat and chair forms. For simplicity and convenience, the carbon atoms of the ring and their associated hydrogen’s are often omitted from the Haworth representation (Fig. 5-7). The cyclic forms of two other common mono saccharides, α-D-ribose and β-D-fructose, are shown in Figure 5-8. It should be noted that while both glucose and fructose are hexoses, glucose (an aldohexose) forms a pyranose whereas fructose (a ketohexose) forms a furanose.