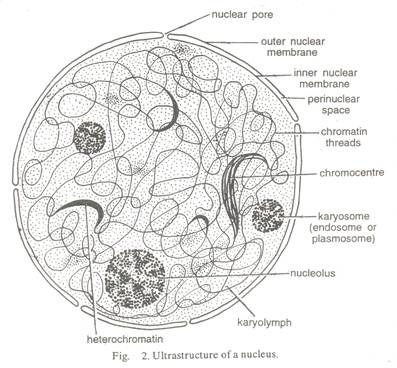
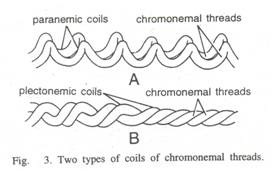
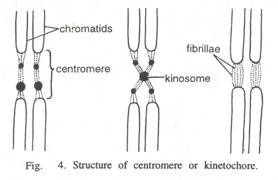
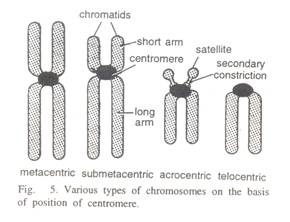
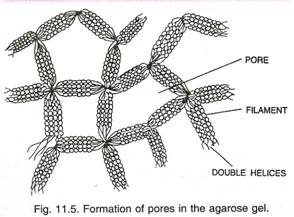
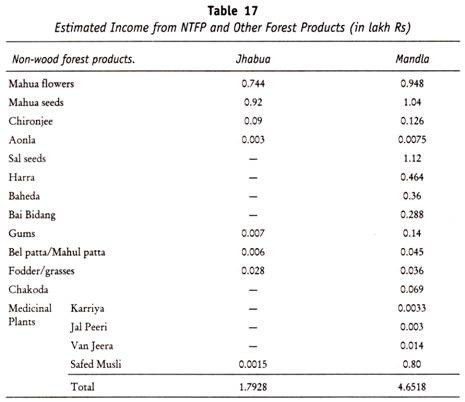
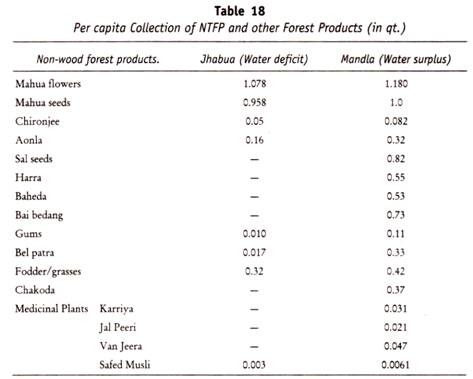
Here is a list of fifteen main chemical agents that can kill infection causing organisms:- 1. Preservatives 2. Acids 3. Nitrates and Nitrites 4. Alkalies 5. Salts 6. Reducing Agents 7. Oxidizing Agents 8. Phenols and Cresols 9. Alcohols 10. Glycols 11. Dyes 12. Mercury Compounds 13. Soaps 14. Surface-active Agents and Synthetic Detergents 15. Sulfonamides.
Chemical Agent # 1. Preservatives:
The use of chemical additives is widespread and represents an important means of preserving foods and other products. The choice of the chemical preservative used depends on the nature of the food and the likely spoilage microorganisms.
Although there is great concern today over the addition of any chemicals to foods because of the finding that some chemicals that have been used as food additives, such as red dye number 2, are potential carcinogens, it must remembered that the effective preservation of food prevents spoilage and the transmission of food-borne diseases.
In the United States the federal Food and Drug Administration is responsible for determining and certifying the safety of food additives and must approve any chemicals that are added to foods as preservatives.
Chemical Agent # 2. Acids:
Various low molecular weight carboxylic acids are inhibitors of microbial growth. Lactic, acetic, propionic, citric, benzoic, and sorbic acids or their salts are effective food preservatives. An examination of the lists of food additives in the various foods in your pantry will rapidly convince you of the wide use of organic acids as preservatives.
The effectiveness of a particular organic acid depends upon the pH of the food. For example, at the same pH the citric acid is less effective than lactic acid, which in turn is less effective than acetic acid.
i. Hydrochloric Acid and Sulphuric Acid:
The germicidal efficiency of acids is proportional to the hydrogen-ion concentrations of their solutions. A strong acid (HCl, H2SO4) is, therefore, more germicidal than a weak acid (lactic, acetic). Winslow and Lochridge (1906) found that it required a 0.0077 N solution of HCl or a 0.0096 N solution of H2SO4 to produce a 99 per cent reduction in an E. coli population in 40 min. Since the degree of dissociation is greater with HCl than with H2SO4, the final hydrogen-ion concentrations of the two solutions are practically the same.
ii. Propionates:
Propionates are primarily effective against filamentous fungi. The calcium and sodium salts of propionic acid are used as preservatives in bread, cake, and various cheeses, and because propionates are effective inhibitors of rope formation, they are added to bread dough and milk. Besides their intentional addition to various foods, propionates form naturally during the production of Swiss cheese and act as a natural preservative.
iii. Lactic and Acetic Acid:
Lactic and acetic acids also are effective preservatives that form naturally in some food products. Cheeses, pickles, and sauerkraut contain concentrations of lactic acid that normally protect the food against spoilage. Vinegar contains acetic acid, an effective inhibitor of bacterial and fungal growth.
Acetic acid is used to pickle meat products and is added as a preservative to various other products, including mayonnaise and catsup. Both of these preservatives, however, will prevent surface fungal growth on a food only if molecular oxygen is excluded.
iv. Benzoates:
Benzoates, including sodium benzoate, methyl p-hydroxybenzoate (methylparaben), and propyl-p- hydroxybenzoate (propylparaben) are extensively used as food preservatives. Benzoates are used as preservatives in such products as fruit juices, jams, jellies, soft drinks, salad dressings, fruit salads, relishes, tomato catsup, and margarine. They are also used as preservatives in a great variety of pharmaceutical preparations.
v. Sorbic Acid:
Sorbic acid, used primarily as calcium, sodium, or potassium salts (for example, sodium sorbate) is more effective as a preservative at pH 4-6 than the benzoates. Sorbates inhibit fungi and bacteria, such as Salmonella, Staphylococcus, and Steptococcus species. Sorbates are frequently added as preservatives to cheeses, baked goods, soft drinks, fruit juices, syrups, jellies, jams, dried fruits, margarine, and various other products.
vi. Boric Acid:
Boric acid is used as a preservative in eyewash and other products. The limited toxicity of boric acid to human tissues makes it suitable for such applications. Boric acid is also used in urine collection jars to prevent bacterial growth between the time of collection and analysis.
Chemical Agent # 3. Nitrates and Nitrites:
Nitrates and nitrites are added to cured meats to preserve the red meat colour and protect against the growth of food spoilage and poisoning microorganisms Nitrates are effective inhibitors of Clostridium botulinum in meat products such as bacon, ham, and sausages.
Recently, however, there has been great concern over the addition of nitrates and nitrites to meats because these salts can react with secondary and tertiary amines to form nitrosamines, which are highly carcinogenic.
Chemical Agent # 4. Alkalies:
The disinfecting action of alkalies is dependent upon the presence of hydroxyl ions. The greater the degree of dissociation, the more effective the germicidal action. Alkalies that are especially toxic to bacteria include KOH, NaOH, LiOH, and NH4OH. Of these, KOH shows the greatest germicidal action by virtue of its greater degree of dissociation; NH4OH shows the smallest because it is the least ionized.
There are some exceptions to the above rule. Barium hydroxide, Ba (OH)2 for example, is less dissociated than KOH, yet it is considerably more toxic. This is due to the high toxicity of the bariumion. The combined action of the barium and hydroxyl ions produces a greater germicidal action than that exhibited by either ion acting alone. Hydrogen ions exert a greater toxic effect than an equivalent number of hydroxyl ions.
Chemical Agent # 5. Salts:
Cations exert a peculiar and characteristic effect on the viability of bacteria. In general, cations in low concentrations tend to stimulate bacterial growth; in high concentrations, they are inhibitory and ultimately toxic.
Sherman and Holm (1922) found low concentrations of NaCl to produce an accelerating effect on the growth of E. coli. The optimum stimulating action occurred at a salt concentration of about 0.2 M. The optimum pH for growth in both the control and salt media appeared to be about 7.8.
They also reported that E. coli rarely grew in a 1 per cent peptone medium at pH 4.8, but grew quite readily in the same medium to which was added NaCl to make a 0.2 M solution. The NaCl produced a widening effect upon the pH limit of growth. This widening effect was even more pronounced upon the growth of other bacterial species.
Hotchkiss (1923) combined different cations with the same anion (chloride) and tested their effect on the growth of E. coli. The salts could be divided into two groups on the basis of their toxicity. The salts in group 1 showed no growth of E. coli in concentrations of 2 to 0.05 M; those in group 2 prevented growth in dilutions of 0.01 to 0.00001 M. The salts in group 1 are of common occurrence in the protoplasmic environment and are considered nontoxic.
The salts are grouped as follows:
Group 1 – Chlorides of Na, K, Li, NH., Sr, Mg, Ca, Ba, Mn, Ti, and Sn.
Group 2 – Chlorides of Ni, TI, Cu, Fe, Zn, Co, Pb, Al, Ce, Cd, and Hg.
Studies on the chlorides of Na, K, NH4 and Li showed that maximum growth occurred at a salt concentration of 0.25 M after an incubation period of 3 days. Salt concentrations above or below 0.25 M showed a decreased growth of the organisms.
In general, the bivalent salts in group 1 showed a greater toxicity than the monovalent salts. The optimum growth concentration ranged from about 0.05 to 0.025 M. The salts in group 2 exhibited a greater degree of toxicity to E. coli.
Salts which are toxic become stimulating in higher dilutions. However, the toxic effects have been studied to a greater extent. Salts of heavy metals, particularly mercury and silver, are toxic to bacteria in relatively low concentrations. The toxicity of solutions of HgCl2 is due to the concentration of Hg ions in solution.
The greater the concentration of free Hg ions, the more efficient the germicidal action. Mercury salts of the organic acids, e.g., mercury acetate, which show a low degree of dissociation, exhibit a much weaker germicidal action.
The toxic action of salts of mineral acids may be due to the cation, to the anion, to the molecule taken as a whole, or to all three. In order to determine to which component the action is due tests have been made in which one cation was combined with different anions and different cations combined with the same anion.
Winslow and Hotchkiss (1922) tested a number of cations on various bacteria and reported that, in general, the toxicity of cations increased with valence. They arranged the cations in order of increasing toxicity as follows – K, Na, NH4, Li, Sr, Mg, Ba, Ca, Mn, Ti+++ Sn, Ni, Tip–, Zn, Cu, Fe++ Fe+++ Co, Pb, Al, Ce, Cd, Hg.
Holm and Sherman (1921) determined the growth rates of E. coli in a peptone solution to which were added various Na salts. They concluded that the chloride ion showed the least toxicity and the fluoride ion the most. The CI, I, NO3, SO4, PO, and lactate ions accelerated growth, whereas the oxalate, acetate, citrate, and fluoride ions exhibited an inhibitory effect.
Different species of bacteria vary considerably in their susceptibility to the same ion. Eisenberg (1919) showed that Bacillus anthracis was quite resistant to the action of the fluoride, iodide, and oxalate ions; Corynebacterium diphtheriae to tellurates, tellurites, nickel, and copper; Salmonella typhosa to strontium salt; the pneumococcus to ferricyanides, tellurites; etc. In other words, organisms may be classified on the basis of their susceptibility to the various ions.
Another point to consider in making a study of the action of ions on bacteria is the composition of the culture medium. Salts exhibit a greater germicidal action in distilled water than the same concentration in a protein-containing medium. This is due to a chemical reaction between the salts and the proteins, resulting in a decreased concentration of ions in the medium.
In general, Gram-positive organisms are more sensitive to various ions than Gram-negative bacteria. The same holds true for the action of various dyes on Gram-positive and Gram-negative bacteria.
Chemical Agent # 6. Reducing Agents:
Some compounds produce a germicidal action by virtue of their powers of reduction. Sulfurous acid, sulfites, ferrous compounds, and formaldehyde act in this manner. Formaldehyde is a very efficient germicide, being effective against both vegetative cells and spores. A 5 per cent solution of formalin (a 37 per cent solution of formaldehyde gas in water) destroys anthrax spores in 1 to 2 hr.
Chemical Agent # 7. Oxidizing Agents:
Compounds that give up oxygen freely or are capable of releasing oxygen from other compounds have been used as germicides. Such agents produce their toxic effects by the process of oxidation. Among these may be mentioned hydrogen peroxide, potassium permanganate, the halogens (chlorine, bromine, iodine), and certain compounds containing these elements, such as hypochlorous acid (HCIO) and hypochlorites; bleaching powder, CaCI (OCl); chloramine, CH3 C6H4. SO2NNaCl.3H2O; and dichloramine, CH3. C6H4 . SO2NCl2.
Hydrogen peroxide is an active oxidizing agent, being easily decomposed into water and oxygen. The commercial solution of H2O2 (3 per cent) is said to be capable of destroying anthrax spores in 1 hr.
Potassium permanganate was at one time employed to a considerable extent for the destruction of bacteria. Its action is increased in acid solution. A solution containing 1 per cent KMnO4 and 1.1 per cent HCI in water is said to destroy anthrax spores in 30 sec. The salt promptly reacts with organic matter, being changed to insoluble MnO2, a brown-staining compound. For this reason, the use of KMnO4 as a germicide has been largely discontinued.
Bleaching powder is probably the most important oxygen compound of the halogens.
When this compound is dissolved in water, it is said to break down as follows:
2CaCI(OCI) + 2CO2 + 2H2O <=> CaCl2 + Ca(HCO3)2 + 2HCIO
The HCIO then breaks down to hydrogen chloride and oxygen:
2HCIO <=> 2HCI + O2
If chlorine gas is employed, the reactions are:
Cl2 + H2O <=> HCl + HCIO
2HCIO <=> 2HCl + O2
Compounds containing active chlorine attached to a nitrogen atom of the general formula R2 = N—CI and R—N = Cl2 are also strongly germicidal, the activity being directly proportional to the extent to which reactions of hydrolysis proceed in solution –
The active agent is HCIO:
2HCIO <=> 2HCl + O2
Bromine added to water reacts in the following manner:
or
The mechanism of action of iodine appears to be different from that of chlorine and bromine. An aqueous solution of iodine at pH 8 or less contains chiefly two forms of iodine, namely, molecular I2 and the triiodide ion, I3–. The I2 is only slightly soluble in water. In the presence of an iodide, such as Nal, the solubility is increased several hundred times, the increase being in the tri-iodide form:
I2 + I– → I3–
Carroll (1955) found the tri-iodide ion to have negligible bactericidal activity. Since iodine for bactericidal studies is used in high dilutions, the tri-iodide ion dissociates into diatomic iodine and iodide ion unless the relative concentration of iodide (Nal, KI, etc.) is kept at a high level.
Gershenfeld and Witlin (1949) found that solutions containing free iodine displayed more effective antibacterial activity against Staphylococcus aureus than did chlorine or bromine, either in the presence or in the absence of organic matter.
Knox et al. (1948) reported that chlorine, in bactericidal concentrations or less, inhibited various sulfhydryl enzymes and other enzymes sensitive to oxidation. Inhibition of essential enzymes caused death of the cells.
Inhibition of glucose oxidation was paralleled by the percentage of bacteria killed. The aldolase of Escherichia coli was shown to be one of the essential enzymes of glucose oxidation sufficiently sensitive to chlorine to account for its bactericidal effect.
Iodine is a suitable agent for the emergency disinfection of water supplies. Chang and Morris (1953) reported that iodine in a concentration of 5 to 10 p.p.m. is effective against all types of water-borne pathogenic organisms within 10 min. at room temperature.
For this purpose iodine has the following advantages:
(1) Its germicidal action is less dependent on pH, temperature, and time of contact;
(2) Nitrogenous impurities do not impair its usefulness; and
(3) Side reactions leading to consumption of the germicide are less marked for iodine than for chlorinous disinfectants.
Chemical Agent # 8. Phenols and Cresols:
The phenols and cresols are very efficient germicides in fairly concentrated solutions. Phenol is soluble in water, but most of the other members of the group are only slightly soluble. However, they may be held in suspension by mixing with soap, by which procedure colloidal solutions are obtained.
The emulsification of disinfectants only slightly soluble in water results in the formation of more potent germicidal preparations. In the emulsified state, the particles of germicide are adsorbed onto the surface of the emulsifying agent (soap), resulting in an increased concentration in the vicinity of the bacteria.
The emulsified disinfectants are more active when freshly prepared. After a few days, the activity decreases, probably owing to a change in their colloidal state. An important commercial disinfectant of this type is compound solution of cresol, known under the trade name of Lysol.
It is usually stated that phenols and cresols act on proteins with the formation of insoluble proteinates. This results in a precipitation of the proteins of the protoplasm. Kojima (1931) opposed the theory of direct coagulation of the bacterial proteins. He found that the strength of phenol that was required to destroy bacteria failed to coagulate egg albumin.
Reichel (1909) believed that the action was more physical than chemical, the phenol being capable of dissolving in coagulated proteins and in lipoids, fats, and the cytoplasm of bacteria. The germicidal action was due to its ability to penetrate the cell in the form of a colloidal solution.
Chemical Agent # 9. Alcohols:
Absolute alcohol is generally not germicidal or only slightly so. On the addition of water, however, the compound shows a marked germicidal effect. Its maximum germicidal efficiency is exhibited in a concentration of 70 per cent by weight (77 per cent by volume).
Smith (1947) found alcohol to be an effective germicide against Mycobacterium tuberculosis. The organism was killed in 15 to 30 sec. by absolute, 95 per cent, and even 70 per cent ethyl alcohol. Ninety-five per cent alcohols was found to be best for wet surfaces, 50 per cent for dry, and 70 per cent for wet or dry.
Tanner and Wilson (1943) reported that the germicidal action of aliphatic alcohols increased with the molecular weight as far as the amyl derivative (5 carbon atoms) and decreased through octyl to undecyl alcohol (11 carbon atoms). Since the alcohols decrease in solubility as the molecular weights increase, the higher members of the series are generally not employed as germicidal agents.
Alcohols are believed to act by denaturing proteins. This occurs more readily in the presence of water than in its absence, which explains why absolute alcohol is less bactericidal than mixtures of alcohol and water.
The addition of absolute alcohol to mercuric chloride reduces greatly the germicidal potency of the latter. Mercuric chloride dissolved in 50 per cent alcohol is more germicidal than a corresponding aqueous solution. The same holds true for silver nitrate.
Since the toxicity of these salts is proportional to the concentration of mercury and silver ions, water is necessary for ionization to occur. On the other hand, compounds such as phenol and formaldehyde are less germicidal in the presence of even a small amount of alcohol.
Chemical Agent # 10. Glycols:
Robertson et al. (1948) made a study of a large number of glycols, especially propylene, dipropylene, and triethylene glycols, since these compounds possess certain properties which make them acceptable for use as air disinfectants in atmospheres occupied by human beings.
The formulas are as follows:
They found that the killing effect of the glycols was much less than that of the phenols, halogens, and detergents. Even the most highly bactericidal glycols failed to inhibit the growth of bacteria in concentrations of less than 3 per cent, and the least lethal showed an effect only in solutions above 50 per cent.
However, comparison with ethyl alcohol revealed the fact that this compound was only slightly more effective in preventing the growth of microorganisms than was propylene glycol, the most bactericidal of the nontoxic glycols.
In general, the higher the concentration of glycol, the more rapid the bactericidal action. In 98 per cent solution both propylene and triethylene glycol killed in less than 1 min. and probably in a few seconds. Propylene glycol in concentrations of 70 to 80 per cent appeared to produce equally rapid killing and was found to be the most efficient of the three glycols. The action of dipropylene glycol was less regular.
Bazzicalupo, Portella, and Contieri (1951) reported that, in experimentally infected guinea pigs, subcutaneous treatment with propylene glycol inhibited extensive tuberculous alterations and prolonged the survival time.
It’s in vivo suppression of tuberculosis was equal to that of other substances with in vitro bacteriostatic properties. The antituber-culous power of propylene glycol in vivo was greater when the treatment was begun immediately after infection.
Chemical Agent # 11. Dyes:
Certain coal-tar dyes, notably those of the triphenylmethane group, possess the power of affecting the viability of bacteria. This action was first described as bactericidal because it was believed that in the absence of growth the organisms were killed. It was shown later that the organisms were not always killed but merely prevented from multiplying. Churchman (1912, 1928) applied the term bacteriostasis to describe this condition.
In most cases, selective bacteriostatic action parallels the Gram reaction. This means that those organisms which retain the Gram stain (Gram +) are more susceptible to the action of the above dyes than are the Gram-negative bacteria. Conversely, those organisms which do not retain the Gram stain (Gram -) are more resistant to the action of the above dyes than are the Gram-positive bacteria.
Notable exceptions to the parallelism between bacteriostatic action and Gram reaction are the acid fast organisms Mycobacterium tuberculosis, M. paratuberculosis, M. avium, etc. These organisms are Gram-positive but comparatively resistant to the action of the triphenylmethane dyes.
An increase in basicity of the solution of a basic dye results in an increase in its germicidal power. A decrease in basicity results in a decrease in its germicidal power. Likewise, an increase in acidity of the solution of an acid dye results in an increase in its germicidal power. A decrease in acidity results in a decrease in its germicidal power.
Ingraham (1933) believed that the bacteriostatic effect of crystal violet was due to its property of poising the oxidation-reduction potential in a range too high for cell multiplication to occur. Hoffmann and Rahn (1944) agreed with the findings of Ingraham and extended the work. They found that, above a certain concentration, the dye acted like any other germicide.
The cells died in logarithmic order and in proportion to the dye concentration. The dye was more toxic to young than to old cells, and its toxicity increased only slightly with an increase in pH. This strict germicidal action was probably due to the combination of dye with some indispensable cell constituents.
At lower concentrations, the dye did not give a logarithmic survivor curve and was not influenced by cell age or pH or the dye concentration. Perhaps this unusual effect was due to the unfavourable oxidation-reduction potential poised by the dye. In this range, the cells usually overcame the dye action and multiplied. The dye produced an abnormally long lag period which increased with the dye concentration. The length of the lag phase was inversely proportional to the logarithm of the number of inoculated cells.
Chemical Agent # 12. Mercury Compounds:
Mercurial compounds were among ‘the first to be used for the destruction of bacteria. Of these, mercuric chloride (bichloride of mercury) was believed to be the most potent of the germicidal compounds available. At that time Koch (1881) concluded from his experimental results that bichloride of mercury was very effective against the anthrax organism, killing both vegetative cells and spores in one application in a few minutes.
It was shown later that the bacteria were not necessarily killed but merely prevented from multiplying by traces of the chemical present in the medium. In other words, mercury is a strongly bacteriostatic agent and does not necessarily kill all bacteria in the concentrations generally used.
To eliminate the bacteriostatic action of the mercury adherent to the bacteria and of the small amount carried into the sub-culturing medium in the inoculum, ammonium sulfide was used to inactivate the mercury. By this technique many of the bacteria were shown to be living and capable of multiplying in the sub-culturing medium.
Because of their bacteriostatic action, mercury compounds with dyes or other organic radicals are employed for skin antisepsis. Organomercurials are less toxic and less irritating than the older inorganic chlorides, iodides, and cyanides of mercury.
It is generally believed that the mercurials act by interfering with an essential metabolite. Since compounds containing the -SH radical are essential metabolites, the antibacterial action of mercury may be due to combination with compounds containing the sulfhydryl group. Such action is an inactivation without demonstrable injury to the cell.
Harris, Eisenstark, and Dragsdorf (1954) immersed Escherichia coli in a 0.01 M HgCl2 then treated them with hydrogen sulfide. X-ray diffraction studies showed the presence of intracellular crystals of HgS. The results suggested that the site of cation adsorption was the cytoplasmic membrane of the cell.
Morton, North, and Engley (1948) made a study of the organom-ercurials Metaphen, Merthiolate, and Mercurochrome against Streptococcus pyogenes and showed that they possessed many shortcomings as disinfectants. The organisms, placed in a state of bacteriostasis by these agents, were still capable of producing fatal septicemia when introduced into the animal body.
The fact that bacteria are still infectious when in a state of bacteriostasis is sufficient reason for taking precautions to eliminate the bacteriostatic effect of mercury while testing mercurial compounds in vitro for germicidal activity.
Chemical Agent # 13. Soaps:
It has been known since the beginning of bacteriology that both soft and hard soaps are excellent germicides. Soft soaps are prepared by boiling oils and fats with potassium hydroxide; hard soaps are prepared with sodium hydroxide. The soft soaps are used in preparing liquid soaps and shampoos, whereas the hard soaps are used in preparing soap powders, chips, and bars.
Soap has a number of important physical characteristics. When dissolved in water, it lowers the surface tension, forms colloidal solutions and gels, causes water to wet surfaces more rapidly, gives the solution a soapy or slippery feeling, and has the ability to emulsify and disperse oils and dirt in the solution and thus is able to cleanse.
Various chemicals, such as phenols, cresols, mercuric iodide, mercuric chloride, Metaphen, chloramine, and hexachlorophene, have been incorporated in soaps to enhance their germicidal value. It has been shown that most of these so-called germicidal soaps are no more useful than ordinary soaps for destroying bacteria.
In fact, some materials may lose their germicidal effectiveness in the presence of soap and may even decrease the natural antiseptic properties of soap. For example, soaps containing cresol and phenol are less antiseptic than the cresol or the phenol or the soap alone when used in the same concentrations.
McCulloch (1940) tested a large number of commercial soaps for their action on several strains of Streptococcus agalactiae and concluded as follows:
Solutions of commercial soaps and soap powders, at 40°C., and in the presence of 5 per cent skim milk and 5 per cent broth culture of the organisms, were found to be between two and three times as effective in killing mastitis streptococci in 1 min. as was phenol and were equally as effective as 100 p.p.m. of the most actively germicidal of several hypochlorites tested.
A soap containing cresols was no more germicidal than were the non-medicated soaps, and the soaps containing mercury compounds were only slightly more effective. Soap solutions in the concentrations usually obtained in lathering the hands with soap in warm water are effective disinfectants against mastitis streptococci.
Chemical Agent # 14. Surface-active Agents and Synthetic Detergents:
A surface-active agent has the property of orienting itself between two interfaces in such a way that it brings them into more intimate contact. If the function of the agent is to promote wetting and penetration, it is called a surface active agent.
If the two interfaces are immiscible liquids, the surface- active agent lowers the interfacial tension so that emulsions are formed. Under these conditions the agent is called an emulsifier. If the surface active agent combines both wetting and emulsifying properties to a sufficient degree, it is called a synthetic detergent.
Synthetic detergents, like soaps, consist of a hydrophobic (water-repelling) group and a hydrophilic (water-attracting) group.
The detergent and wetting class of compounds consist largely of anionic agents and possess a negative electrical charge.
They ionize in water like soaps:
The emulsifiers for the most part are nonionic, i.e., do not ionize in water. A typical nonionic agent is glycerol monostearate, an emulsifying agent used in baking, ice cream, and cosmetics –
The cationic agents possess a positive electrical charge, being capable of reversing the action of soaps. They are generally substituted ammonium salts. Some well-known cationics are the quaternary ammonium compounds of the form –
R—N (CH3)3Cl
Probably 75 per cent of the cationic agents are quaternary ammonium salts. Most of the sanitizing and bactericidal agents belong in this group.
Surface-active agents have a tendency to localize in the surface layer or interface of liquids. A surface-active molecule may be diagrammatically represented by a bar for the hydrophobic (fat-soluble) group and a disk for the polar (water-soluble) group, depending upon whether the polar group is at the end or somewhere along the carbon chain.
The surface of a solution containing a surface-active agent is actually altered, being covered with a hydrocarbon film having the thickness of one molecular layer. When an aqueous solution containing a wetting agent is in contact with a lipoidal surface, the hydrophobic group of the wetter is absorbed and the polar group protrudes.
Such a surface is now capable of being wetted by water. For this reason wetting agents lower the surface tension of water. Such solutions can penetrate into openings and cracks, very small spaces, or even into the center of clumps of bacteria. The same solutions without wetters would simply bridge over openings without showing any appreciable penetration.
Surface-active agents are of great importance as additions to germicidal solutions intended for clinical application. They make it possible for such solutions to penetrate into infected tissues, pus, necrotic debris, bacteria, etc.
Chemical Agent # 15. Sulfonamides:
The sulfonamides is a name given to a group of drugs that exhibit bacteriostatic activity in vitro and a bactericidal effect in vivo. The first important member of this group to be widely used clinically is para-amino benzene sulfonamide (the amide of sulfanilic acid), commonly known as sulfanilamide-
A large number of derivatives of sulfanilamide have been prepared by substituting the hydrogen atoms of the amino radicals for other groups or radical’s.
Clinical Uses:
The drugs are useful in a number of diseases and infections, some of which at one time produced a very high mortality rate. Some of these are pneumonia, meningitis, gonorrhoea, infections due to micrococci and hemolytic streptococci, gas gangrene, wound and urinary-tract infections. The derivatives vary in their usefulness to a certain disease.
Some may prove to be of great value; others may be useless. Therefore, it is necessary to select the proper derivative for the disease or infection to be treated. As an example, sulfanilamide proved to be of tremendous value to the troops in the Second World War as a dusting powder to wounds to prevent infection. Each soldier carried a supply at all times as an emergency measure.
Mode of Action:
From the many theories advanced to explain the mode of action of the sulfonamides, the one advanced by Fildes (1940) appears to be the most logical. Woods (1940) showed that p-aminobenzoic acid (PABA) in high dilutions antagonized the action of sulfonamides. Fildes showed that PABA is an essential metabolite normally associated with an enzyme. The sulfonamides displace PABA from its enzyme and thereby stop this essential line of metabolism.
Unless a large enough dose of sulfonamides is administered, the organisms are likely to develop a resistance or fastness to the drug, making it necessary to give much larger doses. Landy, Larkum, and Ostwald (1943) and Landy et al. (1943) found that the development of resistance to sulfonamides by Staphylococcus aureus resulted in an increased synthesis of PABA.
Urea and Its Derivatives:
Urea and some of its derivatives, such as urethane, are highly bacteriostatic and bactericidal for many Gram-negative and a few Gram-positive bacteria. They also potentiate the activity of the sulfonamides, inhibit p-aminobenzoic acid moderately, and increase the solubility of sulfanilamide and sulfathiazole. Because of its ability to dissolve necrotic tissue, urea has been used in combination with the sulfonamides in the treatment of wound infections.
Urea or carbamide has the following structure –
Urethane is the ethyl ester of carbamic acid –
Weinstein (1946) reported that the replacement of the = O group in urea by = S, to give thiourea,
increased markedly its antibacterial activity.