In this article we will discuss about:- 1. Meaning of Stains 2. Purpose of Staining 3. Structural Components 4. Classification.
Meaning of Stains:
A stain is any colouring organic compound that, when combined with another substance, imparts a colour to that substance. The terms ‘dyes’ and ‘stains’ are often used interchangeably by biologists, but they are not the same. The term ‘dye’ is used to refer to a colouring agent that is used for general purposes, whereas the term ‘stain’ is used to refer to that dye which is used for biological purposes.
Most of the stains used, particularly for bacteria, are aniline dyes, so called because their derivation from aniline (C6H5NH2). The most commonly used aniline dyes are crystal violet, methylene blue, basic fuchsin, safranin, eosin, etc.
Purpose of Staining:
Staining is done for any or all of the following three purposes:
(a) To see organism better:
Staining enables to see the organism better in contrast with background.
(b) To differentiate one organism from another:
Some microorganisms take colour under the given staining conditions, some do not. Such differences are particularly evident in staining procedures which are therefore called “differential stains”, the most common differential stains being the Gram- stain and the acid-fast stain,
(c) To determine particular structures:
There are special stains which react only with certain structures of the organism, e.g., spores, cell wall, nuclei, or others. This is why an organism stained with a cell wall-stain shows only the presence or absence of its cell wall.
Structural Components (Nature) of Stains:
Stains (dyes) usually have complex molecular structure and are chiefly benzene derivatives. A stain consists of three constituents: the organic compound containing a benzene ring, the chromophore, and the auxochrome. Thus a stain (Fig. 17.1) may be defined chemically as an organic compound containing both chromophore and auxochrome groups linked to its benzene ring.
The ability of a stain to bind macromolecular cellular components such as proteins or nucleic acids depends on the electrical charge found on the chromogen portion, as well as on the cellular component to be stained.
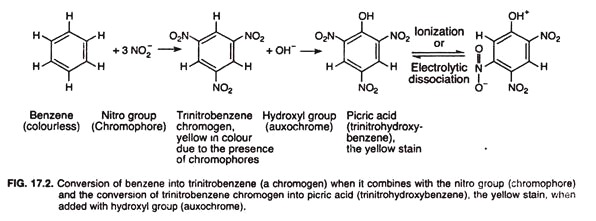
For convenience, when benzene of an organic colourless solvent binds to the nitro group of chromophore, it results in a yellow coloured compound called trinitrobenzene in which three hydrogen atoms in the benzene molecule are replaced by three nitro groups.
Trinitrobenzene is a chromogen but not a stain. If however, another hydrogen atom is replaced by an auxochrome group, such as OH, the compound known as picric acid (trinitrohydroxybenzene) is formed. The picric acid is capable of ionization or electrolytic dissociation to form salt that binds to opposite-charged biological substance (Fig. 17.2).
Thus the picric acid, which is yellow in colour, is a stain (dye). The colour of picric acid is due to the chromophoric nitro group (NO2), and its staining property is due to the auxochromic hydroxyl group (OH), which imparts the molecule the property of ionization or electrolytic dissociation.
Chromophore and Auxochrome:
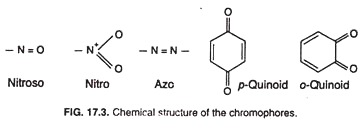
Chromophore (Gk. chroma = colour; phoros = to bear) is such a group with multiple bond that is associated with a compound and produces colour in that compound. It was Otto N. Witt (1876) who first designated the chromophore. Chromophore containing molecule is called chromogen.
The most effective chromophores are nitroso (NO), nitro (NO2), azo (N = N), p-Quinoid, o-Quinoid, etc. The chemical structure of a chromophores is given in Fig. 17.3. The presence of any one of the chromophores in a molecule is usually sufficient to produce colour. Thus nitrobenzene is pale-green, azobenzene is orange-red, p-quinones are yellow, and o- quinones are orange or red.
Auxochromes (Gk. auxein = to increase, chroma = colour) are the groups, while not producing colour themselves, are able to intensify the colour when present in a molecule together with a chromophore. The most effective auxochromes are: —OH, NH2, —NHR. —NR2, CI, and CO2H.
Mordant and Its Function:
Mordant is a substance that forms an insoluble compound with a stain and helps to fix the colour to the cell components. Some stains never stain the cells or its components unless treated with a mordant. The mordant becomes attached to the cell or its components and then combines with the stain to form an insoluble colour complex. This complex is called a lake.
Commonly used mordants are the oxides of aluminium, iron, and chromium. Alizarin is an example of a stain that imparts colour only in collaboration with a mordant. It gives different colours when used with different mordents. It gives red colour with aluminium and tin salts, brownish red colour with a chromium mordant, and black-violets with an iron mordant.
Classification (Types) of Stains:
The stains (dyes) can be classified in various ways on the basis of their origin, purpose of use, staining activity, and charge on their surface.
Different classifications of stains are the following:
1. Classification Based on Origin:
On the basis of their origin the stains can be classified as natural or synthetic.
(i) Natural stains:
These stains are obtained from natural resources directly as natural products. Haematoxylin and carmine are good examples. Haematoxylin is obtained from the heart wood of a tree (Haematoxylon campechianum), whereas carmine is obtained from a cochineal female insect. The natural dyes are used mainly for histological purposes.
(ii) Synthetic stains:
Synthetic stains are artificially produced mainly from fractionation and recombination of coal-tar products hence popularly are called coal-tar dyes. The latter are used mainly for the bacterial stain preparations. Important synthetic stains are safranin, fast green, amiline blue, methylene blue, crystal violet, eosine, acid fuchsin, orange-G, etc.
2. Classification Based on Purpose of Use:
Stains can be categorized as under on the basis of the purpose of their use.
(i) Direct or general stains:
Aniline dyes are able to stain bacteria directly. Exceptions are bacterial spores, e.g., of Bacillus spp. and the bacteria that have waxy coaling on their cell wall, e.g., Mycobacterium spp.
(ii) Indirect stains:
These are the dyes which stain only the background, e.g., nigrosin or India ink used either for observing mucilaginous covering enveloping bacteria (capsules) or certain spores of fungi or cells of unicellular animals.
(iii) Selective stains:
These tains are used for special purposes, to stain particular parts of the organism such as spores, metachromatin granules, flagella, nuclei, etc.
(iv) Differential stains:
These stains are those which enable one to differentiate two different groups of bacteria in a mixture, for instance, gram-positive and gram-negative.
3. Classification Based on Staining Activity:
On the basis of staining activity, the stains can be classified as nuclear stains, cytoplasmic stains, and histological stains.
(i) Nuclear stains:
Nuclear stains are acidic in nature and stain the chromatin materials only. Examples are carmine, haematoxylin, etc.
(ii) Cytoplasmic stains:
These stains are basic in nature and stain the cytoplasm and its inclusions. Fast green, aniline blue, erythrosine, eosin, orange-G, etc. are the examples.
(iii) Histological stains:
Histological stains are those that specifically stain some particular tissues in the sections. Safranin stains the lignified and suberized cell walls.
4. Classification based on Charge:
On the basis of the charge which the stain (dye) molecules possess, they are categorized as acidic, basic, and neutral.
(i) Acidic stains (dyes):
Acidic stains (dyes) are acidic in nature because they possess negative (anionic) charge on their surface on ionization. Acid fuchsin, eosin, and picric acid are examples.
(ii) Basic stains (dyes):
Basic stains (dyes) are basic in nature because they possess positive (cationic) charge on their surface on ionization. Fast green, aniline blue, methylene blue, crystal violet, safranin, etc. are examples.
(iii) Neutral stains (dyes):
Neutral stains (dyes) are formed by the combination of acidic and basic stains in aqueous form. The colouring matter in neutral stains is present in both the anionic and the cationic groups. Therefore, these dyes are neither acidic nor basic. Neutral red is an example.
Acidic and Basic Stains (Dyes):
All stains (dyes) used to stain bacteria are synthetic products because they are artificially produced mainly from fractionation and recombination of coal-tar (aniline) and hence are generally called coal-tar dyes or aniline dyes. Although the synthetic stains (dyes) vary greatly in their chemical nature and staining properties they are, for practical purposes, often divided as acidic (dyes) and basic stains (dyes).
1. Acidic Stains (Dyes):
The acidic stains (dyes) are anionic (negative) and ionize to impart a negative charge on the chromogen portion. An acidic stain (dye), therefore, has a strong affinity for the cationic (positive) constituents of the cell.
These stains (dyes) are used to stain the cytoplasmic components because the proteins, the positively charged cytoplasmic components, readily bind to and accept the colour of the negatively charged chromogen of these stains. Acid fuchsin, eosin, picric acid, etc. are examples. Picric acid, for example, produces an anionic chromogen on ionization as illustrated.
2. Basic Stains (Dyes):
The basic stains (dyes) are cationic (positive) and ionize to provide a positive charge on the chromogen portion. A’ basic stain (dye), therefore, has a strong affinity for the anionic (negative) constituents of the cell.
These stains (dyes) are used to stain the negative charged cellular components (e.g., nucleic acids) because they readily bind and accept the colour of the positively charged cationic chromogen of a basic stain. Methylene blue, crystal violet, safranin, etc. are the basic dyes. Methylene blue is actually a salt (methylene blue chloride) and produces a cationic chromogen as illustrated.
Basic stains (dyes) are more commonly used for bacterial staining. The presence of a negative charge on the bacterial surface acts to repel most acidic stains and thus prevents their penetration into the bacterial cell.
3. Salts of Acidic and Basic Stains (Dyes):
Use of terms ‘acidic’ and ‘basic’ stains (dyes) does not mean that the stains in question are free acids or free bases. The free colour acids and bases are often insoluble in water and rarely possess appreciable staining action, i.e., the colours do not “stick”. The salts of these- compounds, on the other hands, are more soluble, penetrate better, and stain permanently, and they are the true stains.
An acidic stain is the salt of a colour acid, whereas a basic stain is the salt of a colour base. In other words, acid dyes owe their coloured properties to the anion and the basic dye to the cation. However, important salts of acidic and basic stains are shown in Fig. 17.4.