Lipids are heterogeneous group of water insoluble compounds which are oily or greasy in consistency but soluble in non-polar solvents like ether, chloroform, benzene etc.
For examples, fatty acids, fats, oils, waxes, certain vitamins and hormones are considered as lipids. Lipids are composed of C, H, O, like carbohydrates but poor in oxygen and therefore require more oxygen for oxidation to release energy.
Classification:
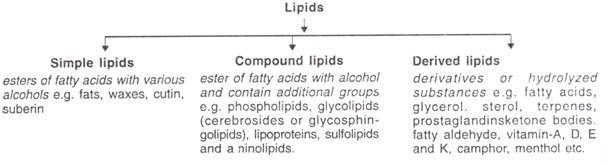
Bloor (1943) coined the term ‘lipid’ and classified them into three types: Simple, Compound and Derived.
(A) Fatty acids:
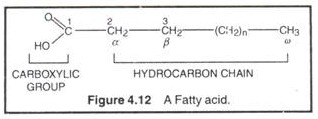
Fatty acids are monocarboxylic acids (R-COOH) with long hydrocarbon chains. Fatty acids don’t occur free in nature; rather occur as esters in natural fats and oils. The fatty acid is called an acyl group when it is a part of ester. In biological systems, fatty acids usually contain an even number of carbon atoms, typically between 14 and 24. The most common fatty acids have 16-18 carbon and 0-3 double bonds. For example, plamitic (C16), stearic (C18), oleic (C18), linoleic (C18) etc.
Fatty acids are amphipathic molecules because their carboxylic groups (-COOH) are hydrophilic or polar and the hydrocarbon chains are hydrophobic or non-polar. In physiological conditions, fatty acids found in ionized forms. For example, palmitic acid exist in form of palmitate and so on.
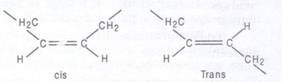
The carbon atoms of fatty acids are numbered from the carboxyl carbon (C-l). The C-2 and C3 are called α-carbon and/β-carbon respectively. The methyl carbon at the end is called ω-carbon. Fatty acids are of 2 types, saturated and unsaturated. Saturated fatty acids contain no double bonds (saturated in hydrocarbon chain, e.g., CH3 (CH2)14COOH (palmitic acid), CH3 (CH2)16COOH (stearic acid), CH3 (CH2)18 carboxylic COOH (arachidonic acid) etc. They have higher group melting points and are solid at room temp. Unsaturated fatty acids contain one or more double bonds (unsaturated) in hydrocarbon chain. A double bond in an unsaturated fatty acid has two possible configurations, cis or Trans. The double bonds in most unsaturated fatty acids have the cis orientation that introduces a bend or kink in the hydrocarbon side chain.
Essential Fatty acids (EFA; Durr and Burr, 1930):
Bacterial and plants can synthesize all the required fatty acids. But animals can’t synthesize 3 polyunsaturated fatty acids – linoleic, linolenic and arachidonic acids – that are required for growth and synthesis of prostaglandins. If linoleic acids are sufficiently available in the diet then other two EFA can be synthesized from it. EFAs are popularly known as Vitamin F.
Importance of Fatty acids:
1. Fatty acids are fuel molecules, stored as triglycerides in the fat-cells (adipose cells). Under the influence of hormone adrenaline adipose cells hydrolyze the triglycerides into free fatty acids that are released into the blood. The complete oxidations of 3 fatty acids of a triglyceride release 9 Kcal/g, in contrast to carbohydrate and protein that release 4 Kcal/g.
2. They are the building blocks of phospholipids, glycolipids and lipoproteins that found in the biological membranes.
3. Eicosanoids (prostaglandins, thromboxane’s and leukotriene’s) are the polyunsaturated fatty acids, which serve as hormones.
4. Fatty acid derivatives act as intracellular messengers, e.g. IP3 (inositol 1, 4, 5-triphosphate) and DAG (diacylglycerol).
5. Deficiency of EFA (essential fatty acids) in human diet causes sterility, kidney failure, skin lesions like phrenoderma (hard skin), eczema etc.
6. Provide the insight to many diseases like obesity atherosclerosis etc.
7. Hydrogenation of unsaturated fatty acids converts oil to solid fat at room temperature. This is the basis of manufacturing edible vanaspati ghee or margarine from inedible and cheap cotton seed oil.
8. Physicians recommend taking PUFA to those having high blood cholesterol or cardiovascular disease.
9. The cleaning action of soap is due to reduction of surface tension of water by fatty acids
(B) Fats (= True fats, neutral fats, triglycerides or triacylglycerol)
They are the unchanged ester of 3 fatty acids and a gtycerol. When 2 or 1 fatty acids esterified with glycerol, then they are called mono-glycerides respects. In pure fats all the 3 fatty acids are similar e.g tripalmitin, tristearin. In mixed fat (triglyceride) all the three or at least one or 2 fatty adds are dissimilar e.g. butter.
Triglycerides are high efficiency storage fuel. In mammals, triglycerides are deposited in the form of large fat globules in the cytoplasm. In migratory birds, the triglycerides are stored under the skin, in muscle, in abdominal cavity & in liver. About two-thirds of this stored fat is consumed in the long flight over water. Triglycerides store six time as much energy as does glycogen because they are deposited in highly reduced and anhydrous form.
Hence, in evolutionary process triglycerides were selected over glycogen as the major energy reservoir. In a typical 70kg man, triglycerides constitute about 11kg of total body weight with a fuel reserve of 105kcal. If this amount of energy is stored in the form of glycogen, his total body weight would be 55kg greater.
Properties of Fats:
1. The fats of animal and plant origin are mixtures of different triglycerides. Plant fats have more unsaturated fatty acids and animal fats have saturated fatty acids. For commercially used fats are different into hard fats (animal fats) and oils (vegetable fats). Fats are solid at room temperature (20°C) but when liquid at 20°C called oils.
2. The property of fat depends upon chain length and their degree of saturation. Short chain length and un-saturation of fatty acids lower the melting point and increase the fluidity.
3. Hydrolysis: Lipases hydrolase the ester bonds so that a triglyceride forms 3 fatty acids and 1 glycerol. It is the basis of fat digestion.
4. Oxidation: On the outer mitochondrial membrane fatty acids first linked to coenzyme A by the enzyme fatty acid thiokinase. This is called fatty acid activation. Subsequently the activated fatty acids enter into the mitochondrial matrix where /β-oxidation (oxidation at /β-carbon) takes place to liberate energy.
5. Saponification: It is the process of formation of soap (metallic salts of fatty acids) by boiling a fat or oil with alkali like KOH, NaOH etc.
6. Saponification number: It is the number of milligrams of KOH required to saponify 1 gm of a given fat or oil. Saponification gives an idea about the molecular weight or chain length of the fatty acids of a fat. A fat having smaller fatty acids have higher saponification.
7. Rancidity: It is the development of unpleasant odour and taste of a fat or oil when kept for a long time, or exposed to air, moisture, heat etc. Rancidity is caused by microbial degradation of fatty acids, hydrolysis of a fat and formation of peroxides at the double bonds. Vegetable fats can be preserved for longer period than animal fat. This is because vegetable fats (oils) contain antioxidants like Vitamin E, Phenols etc. which prevent rancidity.
8. Acid Number: It is the number of milligrams of KOH required to neutralize the free fatty acids present in 1 gm of fat. The acid number indicates the degree of rancidity of a given fat. Higher the acid number, greater is the rancidity or fat.
9. Iodine Number: It is the number of grams of iodine absorbed by 100 gm. of fat. It indicates the degree of un-saturation of fat.
(C) Waxes:
Waxes are esters of long chain fatty acids with monohydric alcohols. The fatty acids and alcohols in the waxes vary from 24 to 36 carbons. Waxes form water-proof coating on the surface of plants and animals.
The well known waxes are as follows:
i. Bee-Wax:
Secreted from the abdominal glands of worker bees to build honeycombs. It is the ester of palmitic acid and hexacosonol.
ii. Carnauba wax:
It occurs as a coating on the leaves of Carnauba palm tree of Brazil (Copernicia prunifera). It is used in automobile polishes.
iii. Spermaceti (Sperm oil):
It is the hardest known wax obtained from the head of sperm whale. It is used as lubricating wax.
iv. Lanolin (wool fat):
Secreted from the cutaneous glands of fur bearing animals. It is closing similar to sebum.
v. Wax-D:
It is secreted by tuberculosis and leprosy causing bacteria. It is a measure cause of the diseases.
vi. Cerumen (ear wax):
Secreted from the ceruminous glands of external auditory canal.
vii. Paraffin wax:
It is obtained from petroleum. Candles are prepared from paraffin wax and stearic acid.
(D) Cutin:
It is formed by cross-esterification and polymerization of hydroxyl fatty acids. Along with wax (cuticular wax), cut in form cuticle on leaf epidermis which check transpiration. The cuticle is frequently covered by a layer of epicuticular wax.
(E) Suberin:
It is composed of glycerol and phellonic acid. It is found in the walls of cork cells and from Casparian strip of root endodermis. It is water impermeable and checks infection in plants.
(F) Phospholipids (major membrane lipids):
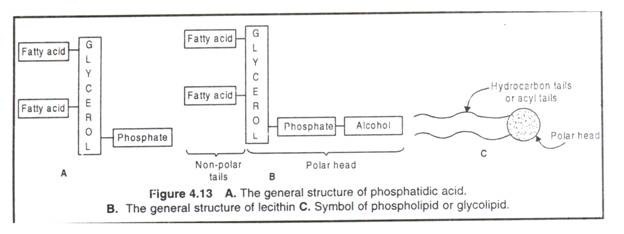
Phospholipids are the esters of fatty acids with glycerol or sphingosine containing an esterified phosphoric acid. The phospholipids derived from glycerol (a 3-C alcohol) are called phosphoglycerides or glycerophospholipids; when derived from sphingosine (CI8 amino alcohol), they are called sphingolipids, e.g. sphingomyelins, cerebrosides and gangliosides.
The phosphatidic acid (diaclylglycerol-3 phosphate) is the simplest phosphoglyceride from which others are derived such as phosphatidylcholine (lecithin), phosphatidylethanolamine (cephalin), phosphatidylserine, phosphatidylinositol and plasmalogens.
In phosphatidic acid C-l and C-2 of glycerol are esterified to two fatty acids and the C-3 is esterified to phosphoric acid. The phosphate group of phosphatidic acid becomes esterified to several nitrogenous alcohols like choline, ethanolamine, serine, inositol etc.
Functions of phospholipids:
1. Phosphatidic acid is an intermediate in the synthesis of triglycerides and phospholipids.
2. Phosphatidic acid is found in bacterial wall and inner mitochondrial membrane.
3. Lecithin acts as a surfactant that lowers the surface tension of lung alveoli. This prevents the collapsing of alveoli.
4. Phosphatidyl inositol 1, 4, 5-bisphosphate (PIP2) is an important phospholipid of cell membrane which hydrolyzed into 2 internal messengers or second messengers in Ca2+ dependant hormone action. They are IP3 and DAG.
5. Plasmalogens are important phospholipids of brain and muscle.
6. Sphingomyelins occur in myelin sheath that surrounds the axon of neuron.
7. The fluidity of the membrane is regulated in different environments. This is possible by changing the nature of fatty acids in the phosphoglycerides. For example, at low temperature bacterial cell membrane contains more unsaturated fatty acids than the bacteria that grow at high temperatures.
Further, at low temperature organisms maintain fluidity by increasing the phosphoglycerides with more unsaturated fatty acids.
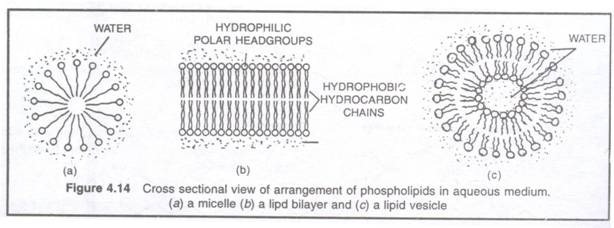
8. Due to amphipathic nature, phospholipids and glycolipids from micelle, lipid bilayer or liposomes (lipid vesicles) in aqueous medium.
(G) Glycolipids:
Composition:
These are conjugated lipids which contain fatty acids, alcohol sphingosine and sugar (galactose). The latter replaces one fatty acid molecule.
Functions:
The glycolipids are components of cell membrane, particularly in myelin sheath of nerve fibers and on outer surfaces of nerve cells. They are components of chloroplast membranes also.
(H) Lipoproteins:
Composition:
These are also conjugated lipids that contain lipids (mainly phospholipids) and proteins in their molecules.
Functions:
Lipoproteins are present in cell membranes. Lipids are transported in the blood plasma and lymph as lipoproteins. Lipoproteins occur in the milk and egg yolk.
(I) Steroids:
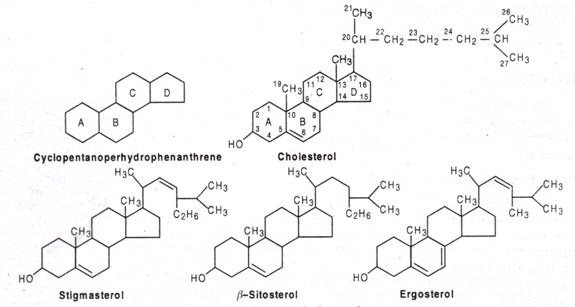
Steroids are the derivatives of 17-C cyclopentanoperhydrophenanthrene structure that consists of 4 fused saturated rings. Out of 4 rings, A, B & C rings are hexane rings (phenanthrene) and D-ring is cyclopentane ring. The methyl (-CH3) groups occur at C-10 and C-13. A side chain is usual at C-17. The steroid hormones are named as estrane (19C, one angular -CH3 group), androstane (19C, 2 angular – CH3 group), pragnane (21C, with 2 angular side chain and a 2C side chain at C-17).
The steroids are of following types:
(a) Sterols:
When the steroids contain one or more -OH group and no carbonyl group, it is called a sterol, e.g. cholesterol, ergosterol, stigmasterol etc. Cholesterol (C27H45OH) found in plasma membrane of animal cells and mycoplasma. It occurs in animal fats (not in plant fats) in form of cholesteryl ester, here -OH of C-3 esterified with fatty acid. It first isolated from gallstones in 1784. Corticoids cholesterol in synthesized in liver and adrenal cortex. Ergosterol (C28 H48 OH) found in the membrane of yeast and fungi. It is the precursor vitamin D. Stigmasterol and β-sterol is the most common membrane sterols in plants.
(b) Bile acids: e.g. Glycocholic acid and Taurocholic acid.
(c) Sex hormones: Testosterone, Estradiol.
(d) Adrenocortical hormones: Corticosterone
(e) Cardiac glycosides: Stropanthin (from stropanthus), digitoxin (from Digitalis)
(f) Anabolic steroids: Synthetic derivatives of testosterone which are used in promoting growth and repair of tissues.
Importance of Steroids:
1. Cholesterol is the precursor of 5 major steroid hormones- Progesterone, Glucocorticoids and Mineralocorticoids.
2. Cholesterol is the precursor of vitamin-D, ecdysone (moulting hormones) bile salts and bile acids.
3. Cholesterol, ergosterol, stigmasterol and β-sterol are important membrane steroids.
4. Diosgenin, a phytosterol extracted from Dioscorea (Yam plant) and is used in male contraceptive pills.
5. Anabolic steroids increase muscle mass, strength and vigor. They are misused by athletes for body building.
6. Brassinosteroids of brassins derived from compesterol which promote growth and differentiation in plants.