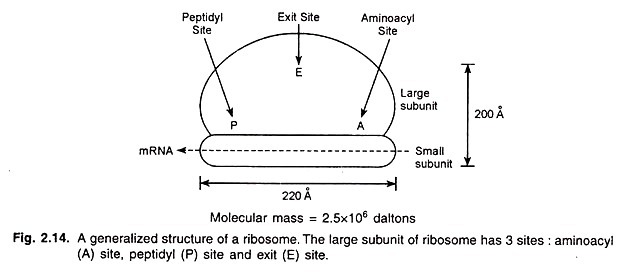
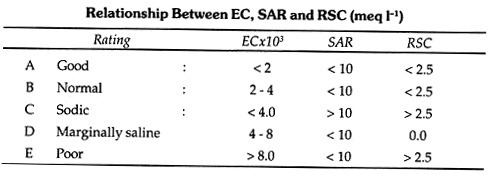
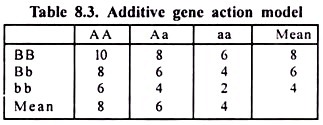
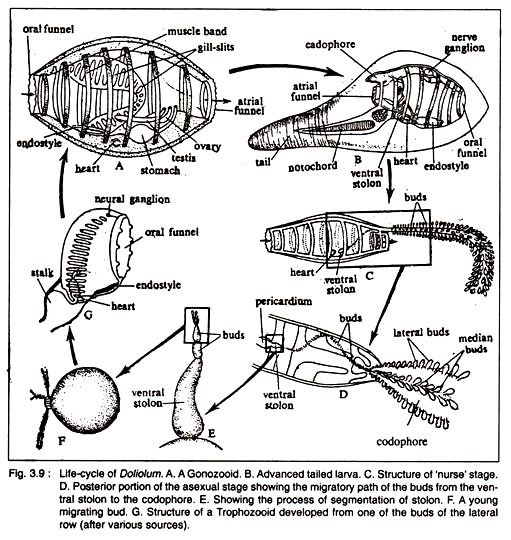
1. Neutral Fats:
The neutral fats, triglycerides, or triacylglycerols are esters of glycerol and three fatty acids and have the general formula shown in Figure 6-2.
In this formula, n, n’, and n” may be the same number or different numbers.
Usually, the fatty acid that is esterified to the first carbon atom of glycerol (i.e., the top carbon in Fig. 6-2) is saturated, whereas the fatty acid esterified to the middle carbon atom is unsaturated.
Either saturated or unsaturated fatty acids are found at the third carbon position. Unlike the fatty acids, the neutral fats are entirely non-polar. Neutral fats, which are often deposited in cells as potential sources of chemical energy, represent the major type of stored lipid; they occur as droplets in the cytoplasm and most of the lipid that is recovered in the soluble phase or cytosol of disrupted cells takes this form. The lipid droplets stored in abundance in muscle cells are clearly seen in Figure 6-3.
2. Glycerophosphatides:
The major members of this group of lipids are derivatives of phosphatidic acid. Phosphatidic acid is similar to a triglyceride except that one of the fatty acids is replaced by a phosphate group (Fig. 6-4). Phosphatidic acid and its derivatives are present in plasma membranes, where they play an active role in membrane function in addition to serving as major structural constituents (Fig. 6-3).
The most common derivatives of phosphatidic acid are phosphatidyl choline (also known as lecithin), phosphatidyl ethanolamine (also known as cephalin), phosphatidyl serine, and phosphatidyl inositol (Fig. 6-5). Free rotation about the single bonds of the glycerol backbone allows the hydrophilic phosphate and its derivatives to face away from the hydrophobic portion, as shown in the structural formulas of Figure 6-5.
Consequently, glycerophosphatides (and other lipids discussed below) are amphipathic, that is, one end of the molecule is strongly hydrophobic (i.e., the end containing the hydrocarbon chains) while the other end is hydrophilic due to the charged nature of the dissociated phosphate group and other substituent’s. In the presence of water, glycerophosphatides can behave in various ways (Fig. 6-6). For example, they can form monolayers (i.e., monomolecular layers) on the water with their polar ends projecting into the water and their hydrophobic ends directed away from the water.
Also, like fatty acids, glycerophosphatides can form micelles with the polar ends of the molecules at the micelle’s surface and the hydrocarbon chains projecting toward the center. However, of special significance is the tendency of glycerophosphatides to form bilayers or bimolecular sheets (Fig. 6-6).
Bilayers formed predominantly from glycerophosphatides are believed to be fundamental to the structure of the plasma membrane, the membranes of the endoplasmic reticulum, and the membranes of vesicular organelles.
Hydrophobic interactions between the tails of the glycerophosphatides are the most important forces promoting the formation of bilayers. The assembly of a bilayer is spontaneous as long as there is adequate lipid present; the spontaneous nature of bilayer formation is reminiscent of the automatic folding of protein molecules that takes place due to the interaction of hydrophobic amino acid side chains.
Liposomes:
When aqueous suspensions of glycerophosphatides are subjected to rapid agitation using ultrasound (i.e., insonation), the lipid disperses in the water and forms liposomes or lipid vesicles (Fig. 6- 7). These liposomes consist of a spherical bilayer of the glycerophosphatide molecules enclosing a small volume of the aqueous medium. If small molecules and ions are initially dissolved in the water, some of these will be enclosed by the liposomes.
The ability of different substances inside or outside the liposomes to traverse the bilayer can be studied experimentally. When this is done, it is found that liposomes exhibit many of the permeability properties of natural cellular membranes.
In recent years there has been considerable interest in using liposomes as vectors for the transfer of drugs, proteins, nucleic acids, ions, and other molecules into cells. The mechanism is illustrated in Figure 6-8. Liposomes are either incubated or formed de novo in aqueous solutions of the substance to be transferred and are then washed free of un-encapsulated material. When mixed with cells in vitro or introduced in vivo (e.g., through intravascular or intra-peritoneal injection), the liposomes fuse with the plasma membranes of the target cells.
The contents of the liposome may enter the cell via either of several routes:
(1) Following fusion, the liposome’s contents may be emptied into the cytosol;
(2) The entire liposome may be endocytosed and degraded, thereby releasing the entrained substances in the cell; and/or
(3) The liposomes may undergo degradation extracellularly, creating a localized high concentration of material that may then be incorporated by the cell. The capability to produce liposomes that differentially bind to the surfaces of specific types of cells and tissues holds great therapeutic promise, for it would make it possible to deliver specific chemical agents to specific tissues.
Plasmalogens:
Plasmalogens are a special class of lipids especially abundant in the membranes of nerve and muscle cells and also characteristic of cancer cells. A plasmalogen is similar to a glycerophosphatide except that the fatty acid at the number 1 position of the glycerol is replaced by unsaturated ether (Fig. 6-9).
Sphingolipids:
Sphingolipids are derivatives of sphingosine, an amino alcohol possessing a long, unsaturated hydrocarbon chain (Fig. 6-10a). The myelin sheath surrounding many nerve cells is particularly rich in the sphingolipid sphingomyelin (Fig. 6-10b). In sphingomyelin, the amino group of the sphingosine skeleton is linked to a fatty acid and the hydroxyl group is esterified to phosphorylcholine.
Glycolipids:
The outer surface of the plasma membrane of most cells is studded with short chains of sugars. These sugars are either parts of glycoproteins or they are attached to membrane lipids, thereby forming glycolipids. Glycolipids in the plasma membrane play vital roles in immunity, blood group specificity, and cell-cell recognition.
The carbohydrates that are found in glycolipids vary from individual mono saccharides (e.g., glucose and galactose) to short, branched or unbranched oligosaccharides. The lipid portion of a glycolipid is similar to sphingosine with the amino group of the sphingosine skeleton acylated by a fatty acid (as in sphingomyelin) and the hydroxyl group associated with the carbohydrate.
The simplest glycolipids are the cerebrosides, which, as their name suggests, are abundant in brain tissue, where they are found in the myelin sheaths and may account for as much as 20% of the sheath’s dry weight. Small amounts of cerebrosides are also found in kidney, liver, and spleen cells. Usually, the sugar of a cerebroside is either glucose or galactose (Fig. 6- 11).
In the gangliosides (also associated with nerve tissue), the carbohydrate portion of the molecule consists of a chain of sugar molecules usually including glucose, galactose, and neuraminic acid. One particular ganglioside, called GM2, may accumulate in the lysosomes of brain cells because of a genetic deficiency that result in the failure of the cells to produce a lysosomal enzyme that degrades this ganglioside. The condition is known as Tay-Sachs disease and frequently leads to paralysis, blindness, and retarded development.