In this article we will discuss about the regulation of levels of transcription by DNA methylation.
In most higher plants and animals, the DNA is often modified after synthesis by the enzymatic conversion of many cytosine bases to 5-methylcytosine bases (Fig. 5.8). The extent of methylation varies from species to species; in mammals, from 2 to 7 percent of the cytosine residues are methylated.
Methyl groups on the 5-carbons of pyrimidines occupy exposed positions within the major grooves of DNA molecules, thus they have the potential to play influential roles in the interactions of DNA with specific proteins.
In fact, studies on the binding of the repressor for the lac operon of E. coli to the lac operator DNA have shown that the addition or removal of a single methyl group can sharply change the affinity of the repressor for the DNA. Thus, the potential regulatory role of 5-methyl groups on pyrimidine bases is well established.
To date, there has been no definitive proof of the role of methylation in the regulation of the expression of any eukaryotic gene. Instead, the arguments for the involvement of methylation in the control of gene expression in eukaryotes are based primarily on three kinds of indirect evidence:
(1) Numerous studies have demonstrated a correlation between the level of gene expression and the degree of methylations, such that low methylation → high gene expression, high methylation → low gene expression.
(2) Methylation patterns are tissue specific, at least in some cases.
(3) The drug (base analog) 5-azacytidine, which can not be methylated after it is incorporated into DNA, has been shown to result in the expression of genes in tissues where they normally are not expressed.
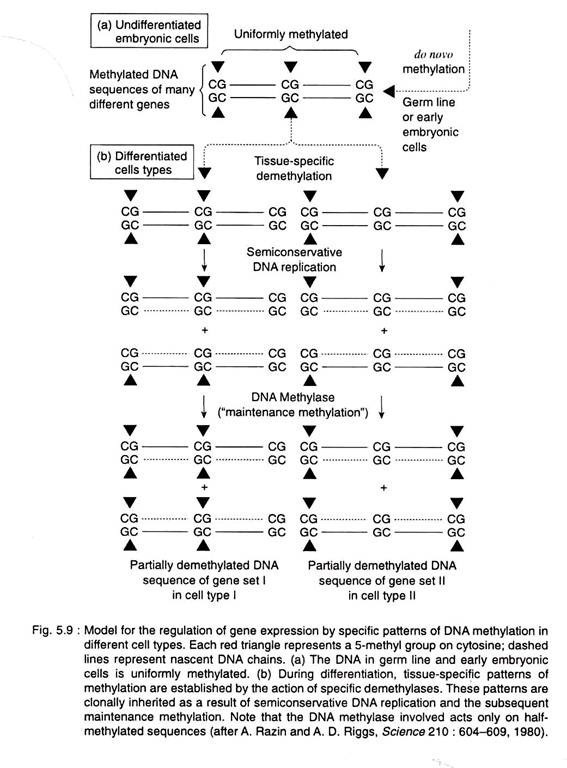
An important aspect of methylation patterns is that they appear to be clonally heritable. Over 90 percent of the methylation in the DNA of most eukaryotes occurs in CG dinu- cleotide sequences, and these sequences are symmetrically methylated, that is
Semiconservative replication of such a symmetrically methylated sequence will yield 2 half-methylated sequences, that is,
In the presence of a DNA methylase that is specific for half-methylated sequences, such methylation patterns will be clonally heritable (Fig. 5.9), that is, once formed, the pattern will be passed on to all the cells of a given lineage. Some bacterial methylases are indeed known to act primarily on half-methylated sites, and considerable evidence points to the existence of eukaryotic methylases with similar properties.
These features of DNA methylation patterns nicely explain their maintenance, once they are formed. However, the key step in any model for the regulation of gene expression or differentiation through DNA methylation involves the formation of the tissue-specific methylation patterns.
The most popular hypothesis is that the patterns are formed during development by tissue specific demethylases, which remove methyl groups from critical sites in genes that are scheduled to be expressed in a particular cell type (Fig. 5.9).
Although this is an attractive model, we should emphasize that it is just that a model. No such tissue-specific demethylase has yet been identified. Recently, the methylation-blocking drug 5-azacytidine has been shown to result in the expression of the fetal (y-hemoglobin) and, to a lesser extent, the embryonic (ε-hemoglobin) β-like hemoglobin genes of anemic adult baboons and adult humans with severe β-thalassemia (an inherited disease characterized by the inability to synthesize the β-hemoglobin chain of adult hemoglobin) and with sickle-cell anemia.
These embryonic and fetal genes are normally not expressed in red blood cells of adults. In one of these studies, the DNA in the region of the γ-hemoglobin and ε-hemoglobin genes was shown to contain fewer methyl groups (to be “undermethylated” or “hypomethylated”) in the red blood cells of the individuals after treatment.
These results not only support the hypothesis that methylation is important in the regulation of gene expression, but also suggest a possible approach to the treatment of these inherited diseases.
Clearly, additional data are needed before any conclusions can be drawn about the role of DNA methylation as a regulatory mechanism in eukaryotes. Nevertheless, the aforementioned and other correlations between methylation patterns and levels of gene expression suggest many interesting questions.
For example, of what importance is the stabilizing effect of methylation on the Z-form of DNA, and is the correlation between under- methylation and nuclease sensitivity of DNA in chromatin biologically significant (see the following two sections)?