This article throws light upon the two methods used for gene transfer in plants.
The two methods are: (1) Vector-Mediated Gene Transfer and (2) Direct or Vector-less DNA Transfer.
Contents
Gene Transfer Methods:
The gene transfer techniques in plant genetic transformation are broadly grouped into two categories:
I. Vector-mediated gene transfer
II. Direct or vector less DNA transfer
The salient features of the commonly used gene (DNA) transfer methods are given in Table 49.1.
Method # I. Vector-Mediated Gene Transfer:
Vector-mediated gene transfer is carried out either by Agrobacterium-mediated transformation or by use of plant viruses as vectors.
Agrobacterium-Mediated Gene Transfer:
Agrobacterium tumefaciens is a soil-borne, Gram-negative bacterium. It is rod shaped and motile, and belongs to the bacterial family of Rhizobiaceae. A. tumefaciens is a phytopathogen, and is treated as the nature’s most effective plant genetic engineer.
Some workers consider this bacterium as the natural expert of inter-kingdom gene transfer. In fact, the major credit for the development of plant transformation techniques goes to the natural unique capability of A. tumefaciens. Thus, this bacterium is the most beloved by plant biotechnologists.
There are mainly two species of Agrobacterium:
i. A. tumefaciens that induces crown gall disease.
ii. A. rhizogenes that induces hairy root disease.
Crown Gall Disease and Ti Plasmid:
Almost 100 years ago (1907), Smith and Townsend postulated that a bacterium was the causative agent of crown gall tumors, although its importance was recognized much later. As A. tumefaciens infects wounded or damaged plant tissues, in induces the formation of a plant tumor called crown gall (Fig. 49.1). The entry of the bacterium into the plant tissues is facilitated by the release of certain phenolic compounds (acetosyringone, hydroxyacetosyringone) by the wounded sites.
Crown gall formation occurs when the bacterium releases its Ti plasmid (tumor- inducing plasmid) into the plant cell cytoplasm. A fragment (segment) of Ti plasmid, referred to as T-DNA, is actually transferred from the bacterium into the host where it gets integrated into the plant cell chromosome (i.e. host genome). Thus, crown gall disease is a naturally evolved genetic engineering process.
The T-DNA carries genes that code for proteins involved in the biosynthesis of growth hormones (auxin and cytokinin) and novel plant metabolites namely opines — amino acid derivatives and agropines — sugar derivatives (Fig. 49.2).
The growth hormones cause plant cells to proliferate and form the gall while opines and agropines are utilized by A. tumefaciens as sources of carbon and energy. As such, opines and agropines are not normally part of the plant metabolism (neither produced nor metabolised). Thus, A. tumefaciens genetically transforms plant cells and creates a biosynthetic machinery to produce nutrients for its own use.
As the bacteria multiply and continue infection, grown gall develops which is a visible mass of the accumulated bacteria and plant material. Crown gall formation is the consequence of the transfer, integration and expression of genes of T-DNA (or Ti plasmid) of A. tumefaciens in the infected plant.
The genetic transformation leads to the formation of crown gall tumors, which interfere with the normal growth of the plant. Several dicotyledonous plants (dicots) are affected by crown gall disease e.g. grapes, roses, stone-fruit trees.
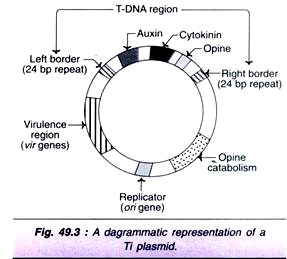
Organization of Ti plasmid:
The Ti plasmids (approximate size 200 kb each) exist as independent replicating circular DNA molecules within the Agrobacterium cells. The T-DNA (transferred DNA) is variable in length in the range of 12 to 24 kb, which depends on the bacterial strain from which Ti plasmids come. Nopaline strains of Ti plasmid have one T-DNA with length of 20 kb while octopine strains have two T-DNA regions referred to as TL and TR that are respectively 14 kb and 7 kb in length.
A diagrammatic representation of a Ti plasmid is depicted in Fig. 49.3. The Ti plasmid has three important regions.
1. T-DNA region:
This region has the genes for the biosynthesis of auxin (aux), cytokinin (cyt) and opine (ocs), and is flanked by left and right borders. These three genes-aux, cyto and ocs are referred to as oncogenes, as they are the determinants of the tumor phenotype.
T-DNA borders — A set of 24 kb sequences present on either side (right and left) of T-DNA are also transferred to the plant cells. It is now clearly established that the right border is more critical for T-DNA transfer and tumori-genesis.
2. Virulence region:
The genes responsible for the transfer of T-DNA into the host plant are located outside T-DNA and the region is referred to as vir or virulence region. Vir region codes for proteins involved in T-DNA transfer. At least nine vir-gene operons have been identified. These include vir A, vir G, vir B1, vir C1, vir D1, D2 and D4, and vir E1, and E2.
3. Opine catabolism region:
This region codes for proteins involved in the uptake and metabolisms of opines. Besides the above three, there is ori region that is responsible for the origin of DNA replication which permits the Ti plasmid to be stably maintained in A. tumefaciens.
T-DNA transfer and integration:
The process of T-DNA transfer and it integration into the host plant genome is depicted in Fig. 49.4, and is briefly described.
1. Signal induction to Agrobacterium:
The wounded plant cells release certain chemicals- phenolic compounds and sugars which are recognized as signals by Agrobacterium. The signals induced result in a sequence of biochemical events in Agrobacterium that ultimately helps in the transfer of T-DNA of T-plasmid.
2. Attachment of Agrobacterium to plant cells:
The Agrobacterium attaches to plant cells through polysaccharides, particularly cellulose fibres produced by the bacterium. Several chromosomal virulence (chv) genes responsible for the attachment of bacterial cells to plant cells have been identified.
3. Production of virulence proteins:
As the signal induction occurs in the Agrobacterium cells attached to plant cells, a series of events take place that result in the production of virulence proteins. To start with, signal induction by phenolics stimulates vir A which in turn activates (by phosphorylation) vir C. This induces expression of virulence genes of Ti plasmid to produce the corresponding virulence proteins (D1, D2, E2, B etc.). Certain sugars (e.g. glucose, galactose, xylose) that induce virulence genes have been identified.
4. Production of T-DNA strand:
The right and left borders of T-DNA are recognized by vir D1/vir D2 proteins. These proteins are involved in the production single-stranded T-DNA (ss DNA), its protection and export to plant cells. The ss T-DNA gets attached to vir D2.
5. Transfer of T-DNA out of Agrobacterium:
The ss T-DNA — vir D2 complex in association with vir G is exported from the bacterial cell. Vir B products form the transport apparatus.
6. Transfer of T-DNA into plant cells and integration:
The T-DNA-vir D2 complex crosses the plant plasma membrane. In the plant cells, T-DNA gets covered with vir E2. This covering protects the T-DNA from degradation by nucleases; vir D2 and vir E2 interact with a variety of plant proteins which influences T-DNA transport and integration.
The T-DNA-vir D2-vir E2 — plant protein complex enters the nucleus through nuclear pore complex. Within the nucleus, the T-DNA gets integrated into the plant chromosome through a process referred to illegitimate recombination. This is different from the homologous recombination, as it does not depend on the sequence similarity.
Hairy Root Disease of A. Rhizogenes — R1 Plasmids:
Agrobacterium rhizogenes can also infect plants. But this results in hairy roots and not crown galls as is the case with A. tumefaciens. The plasmids, of A. rhizogenes have been isolated and characterized. These plasmids, referred to as Ri plasmids, (Ri stands for Root inducing) are of different types. Some of the Ri plasmid strains possess genes that are homologous to Ti plasmid e.g. auxin biosynthetic genes.
Instead of virulence genes, Ri plasmids contain a series of open reading frames on the T-DNA. The products of these genes are involved in the metabolism of plant growth regulators which gets sensitized to auxin and leads to root formation.
Vectors of A. rhizogenes:
As it is done with A tumefaciens, vectors can be constructed by using A. rhizogenes. These vectors are alternate strategies for gene transfer. However, employment of A. rhizogene-based vectors for plant transformation is not common since more efficient systems of A. tumefaciens have been developed.
Importance of hairy roots:
Hairy roots can be cultured in vitro, and thus are important in plant biotechnology. Hairy root systems are useful for the production of secondary metabolites, particularly pharmaceutical proteins.
Ti Plasmid-Derived Vector Systems:
The ability of Ti plasmid of Agrobacterium to genetically transform plants has been described. It is possible to insert a desired DNA sequence (gene) into the T-DNA region (of Ti plasmid), and then use A. tumefaciens to deliver this gene(s) into the genome of plant cell.
In this process, Ti plasmids serve as natural vectors. However, there are several limitations to use Ti plasmids directly as cloning vectors:
i. Ti plasmids are large in size (200-800 kb). Smaller vectors are preferred for recombinant experiments. For this reason, large segments of DNA of Ti plasmid, not essential for cloning, must be removed.
ii. Absence of unique restriction enzyme sites on Ti plasmids.
iii. The phytohormones (auxin, cytokinin) produced prevent the plant cells being regenerated into plants. Therefore auxin and cytokinin genes must be removed.
iv. Opine production in transformed plant cells lowers the plant yield. Therefore opine synthesizing genes which are of no use to plants should be removed.
v. Ti plasmids cannot replicate in E. coli. This limits their utility as E. coli is widely used in recombinant experiments. An alternate arrangement is to add an origin of replication to Ti plasmid that allows the plasmid to replicate in E. coli.
Considering the above limitations, Ti plasmid- based vectors with suitable modifications have been constructed.
These vectors are mainly composed of the following components:
1. The right border sequence of T-DNA which is absolutely required for T-DNA integration into plant cell DNA.
2. A multiple cloning site (poly-linker DNA) that promotes the insertion of cloned gene into the region between T-DNA borders.
3. An origin of DNA replication that allows the plasmids to multiply in E. coli.
4. A selectable marker gene (e.g. neomycin phosphotransferase) for appropriate selection of the transformed cells.
Two types of Ti plasmid-derived vectors are used for genetic transformation of plants— co-integrate vectors and binary vectors.
Co-integrate vector:
In the co-integrate vector system, the disarmed and modified Ti plasmid combines with an intermediate cloning vector to produce a recombinant Ti plasmid (Fig. 49.5).
Production of disarmed Ti plasmid:
The T-DNA genes for hormone biosynthesis are removed (disarmed). In place of the deleted DNA, a bacterial plasmid (pBR322) DNA sequence is incorporated. This disarmed plasmid, also referred to as receptor plasmid, has the basic structure of T-DNA (right and left borders, virulence genes etc.) necessary to transfer the plant cells.
Construction of intermediate vector:
The intermediate vector is constructed with the following components:
i. A pBR322 sequence DNA homologous to that found in the receptor Ti plasmid.
ii. A plant transformation marker (PTM) e.g. a gene coding for neomycin phosphotransferase II (npt II). This gene confers resistance to kanamycin in the plant cells and thus permits their isolation.
iii. A bacterial resistance marker e.g. a gene coding for spectinomycin resistance. This gene confers spectinomycin resistance to recipient bacterial cells and thus permits their selective isolation.
iv. A multiple cloning site (MCS) where foreign genes can be inserted.
v. A Co/E1 origin of replication which allows the replication of plasmid in E. coli but not in Agrobacterium.
vi. An oriT sequence with basis of mobilization (bom) site for the transfer of intermediate vector from E. coli to Agrobacterium.
Production and use of co-integrate vectors:
The desired foreign gene (target-gene) is first cloned in the multiple cloning site of the intermediate vector. The cloning process is carried out in E. coli, the bacterium where the cloning is most efficient. The intermediate vector is mated with Agrobacterium so that the foreign gene is mobilised into the latter.
The transformed Agrobacterium cells with receptor Ti plasmid and intermediate vector are selectively isolated when grown on a minimal medium containing spectinomycin. The selection process becomes easy since E. coli does not grow on a minimal medium in which Agrobacterium grows.
Within the Agrobacterium cells, intermediate plasmid gets integrated into the receptor Ti plasmid to produce co-integrate plasmid. This plasmid containing plant transformation marker (e.g. npt II) gene and cloned target gene between T-DNA borders is transferred to plant cells. The transformed plant cells can be selected on a medium containing kanamycin when the plant and Agrobacterium cells are incubated together.
Advantages of co-integrate vector:
i. Target genes can be easily cloned
ii. The plasmid is relatively small with a number of restriction sites.
iii. Intermediate plasmid is conveniently cloned in E. coli and transferred to Agrobacterium.
Binary vector:
The binary vector system consists of an Agrobacterium strain along with a disarmed Ti plasmid called vir helper plasmid (the entire T-DNA region including borders deleted while vir gene is retained). It may be noted that both of them are not physically linked (or integrated). A binary vector with T-DNA can replicate in E. coli and Agrobacterium.
A diagrammatic representation of a typical binary vector system is depicted in Fig. 49.6. The binary vector has the following components.
1. Left and right borders that delimit the T-DNA region.
2. A plant transformation marker (PTM) e.g. npt II that confers kanamycin resistance in plant transformed cells.
3. A multiple cloning site (MCS) for introducing target/foreign genes.
4. A bacterial resistance marker e.g. tetracycline resistance gene for selecting binary vector colonies in E. coli and Agrobacterium.
5. oriT sequence for conjugal mobilization of the binary vector from E. coli to Agrobacterium.
6. A broad host-range origin of replication such as RK2 that allows the replication of binary vector in Agrobacterium.
Production and use of binary vector:
The target (foreign) gene of interest is inserted into the multiple cloning site of the binary vector. In this way, the- target gene is placed between the right and left border repeats and cloned in E. coli. By a mating process, the binary vector is mobilised from E. coli to Agrobacterium. Now, the virulence gene proteins of T-DNA facilitate the transfer of T-DNA of the vector into plant cells.
Advantages of binary vectors:
i. The binary vector system involves only the transfer of a binary plasmid to Agrobacterium without any integration. This is in contrast to co-integrate vector system wherein the intermediate vector is transferred and integrated with disarmed Ti plasmid.
ii. Due to convenience, binary vectors are more frequently used than co-integrate vectors.
Plant Transformation Technique Using Agrobacterium:
Agrobacterium-mediated technique is the most widely used for the transformation of plants and generation of transgenic plants. The important requirements for gene transfer in higher plants through Agrobacterium mediation are listed.
i. The explants of the plant must produce phenolic compounds (e.g. autosyringone) for activation of virulence genes.
ii. Transformed cells/tissues should be capable to regenerate into whole plants.
In general, most of the Agrobacterium-mediated plant transformations have the following basic protocol (Fig. 49.7)
1. Development of Agrobacterium carrying the co-integrate or binary vector with the desired gene.
2. Identification of a suitable explant e.g. cells, protoplasts, tissues, calluses, organs.
3. Co-culture of explants with Agrobacterium.
4. Killing of Agrobacterium with a suitable antibiotic without harming the plant tissue.
5. Selection of transformed plant cells.
6. Regeneration of whole plants.
Advantages of Agrobacterium- mediated transformation:
i. This is a natural method of gene transfer.
ii. Agrobacterium can conveniently infect any explant (cells/tissues/organs).
iii. Even large fragments of DNA can be efficiently transferred.
iv. Stability of transferred DNA is reasonably good.
v. Transformed plants can be regenerated effectively.
Limitations of Agrobacterium- mediated transformation:
i. There is a limitation of host plants for Agrobacterium, since many crop plants (monocotyledons e.g. cereals) are not infected by it. In recent years, virulent strains of Agrobacterium that can infect a wide range of plants have been developed.
ii. The cells that regenerate more efficiently are often difficult to transform, e.g. embryonic cells lie in deep layers which are not easy targets for Agrobacterium.
Virus-Mediated Gene Transfer (Plant Viruses as Vectors):
Plant viruses are considered as efficient gene transfer agents as they can infect the intact plants and amplify the transferred genes through viral genome replication. Viruses are natural vectors for genetic engineering. They can introduce the desirable gene(s) into almost all the plant cells since the viral infections are mostly systemic.
Plant viruses are non-integrative vectors:
The plant viruses do not integrate into the host genome in contrast to the vectors based on T-DNA of A. tumefaciens which are integrative. The viral genomes are suitably modified by introducing desired foreign genes. These recombinant viruses are transferred, multiplied and expressed in plant cells. They spread systemically within the host plant where the new genetic material is expressed.
Criteria for a plant virus vector:
An ideal plant virus for its effective use in gene transfer is expected to posses the following characteristics:
i. The virus must be capable of spreading from cell to cell through plasmodesmata.
ii. The viral genome should be able to replicate in the absence of viral coat protein and spread from cell to cell. This is desirable since the insertion of foreign DNA will make the viral genome too big to be packed.
iii. The recombinant viral genome must elicit little or no disease symptoms in the infected plants.
iv. The virus should have a broad host range.
v. The virus with DNA genome is preferred since the genetic manipulations involve plant DNA.
The three groups of viruses — caulimoviruses, Gemini viruses and RNA viruses that are used as vectors for gene transfer in plants are briefly described.
Caulimoviruses as Vectors:
The caulimoviruses contain circular double- stranded DNA, and are spherical in shape. Caulimoviruses are widely distributed and are responsible for a number of economically important diseases in various crops. The caulimovirus group has around 15 viruses and among these cauliflower mosaic virus (CaMV) is the most important for gene transfer. The other caulimoviruses include carnation etched virus, dahlia mosaic virus, mirabilis mosaic virus and strawberry vein banding virus.
Cauliflower mosaic virus (CaMV):
CaMV infects many plants (e.g. members of Cruciferae, Datura) and can be easily transmitted, even mechanically. Another attractive feature of CaMV is that the infection is systemic, and large quantities of viruses are found in infected cells.
A diagrammatic view of the CaMV genetic map is depicted in Fig. 49.8. The genome of CaMV consists of a 8 kb (8024 bp) relaxed but tightly packed circular DNA with six major and two minor coding regions. The genes II and VII are not essential for viral infection.
Use of CaMV in gene transfer:
For appropriate transmission of CaMV, the foreign DNA must be encapsulated in viral protein. Further, the newly inserted foreign DNA must not interfere with the native assembly of the virus. CaMV genome does not contain any non-coding regions wherein foreign DNA can be inserted. It is fortunate that two genes namely gene II and gene VII have no essential functions for the virus. It is therefore possible to replace one of them and insert the desired foreign gene.
Gene II of CaMV has been successfully replaced with a bacterial gene encoding dihydrofolate reductase that provides resistance to methotrexate. When the chimeric CaMV was transmitted to turnip plants, they were systemically infected and the plants developed resistance to methotrexate.
Limitations of CaMV as a vector:
i. CaMV vector has a limited capacity for insertion of foreign genes.
ii. Infective capacity of CaMV is lost if more than a few hundred nucleotides are introduced.
iii. Helper viruses cannot be used since the foreign DNA gets expelled and wild-type viruses are produced.
Gemini Viruses as Vectors:
The Gemini viruses are so named because they have geminate (Gemini literally means heavenly twins) morphological particles i.e. twin and paired capsid structures. These viruses are characterized by possessing one or two single-stranded circular DNAs (ss DNA). On replications, ss DNA forms an intermediate double-stranded DNA.
The Gemini viruses can infect a wide range of crop plants (monocotyledons and dicotyledons) which attract plant biotechnologists to employ these viruses for gene transfer. Curly top virus (CTV) and maize streak virus (MSV) and bean golden mosaic virus (BGMV) are among the important Gemini viruses.
It has been observed that a large number of replicative forms of a Gemini virus genome accumulate inside the nuclei of infected cells. The single-stranded genomic DNA replicates in the nucleus to form a double-stranded intermediate.
Gemini virus vectors can be used to deliver, amplify and express foreign genes in several plants/ explants (protoplasts, cultured cells). However, the serious drawback in employing Gemini viruses as vectors is that it is very difficult to introduce purified viral DNA into the plants. An alternate arrangement is to take the help of Agrobacterium and carry out gene transfer.
RNA Plant Viruses as Vectors:
There are mainly two type’s single-stranded RNA viruses:
1. Mono-partite viruses:
These viruses are usually large and contain undivided genomes for all the genetic information e.g. tobacco mosaic virus (TMV).
2. Multipartite viruses:
The genome in these viruses is divided into small RNAs which may be in the same particle or different particles, e.g. brome mosaic virus (BMV). HMV contains four RNAs divided between three particles. Plant RNA viruses, in general, are characterized by high level of gene expression, good efficiency to infect cells and spread to different tissues. But the major limitation to use them as vectors is the difficulty of joining RNA molecules in vitro.
Use of cDNA for gene transfer:
Complementary DNA (cDNA) copies of RNA viruses are prepared in vitro. The cDNA so generated can be used as a vector for gene transfer in plants. This approach is tedious and cumbersome. However, some success has been reported. A gene sequence encoding chloramphenicol resistance (enzyme- chloramphenicol acetyltransferase) has been inserted into brome mosaic virus genome. This gene expression, however, has been confined to protoplasts.
Limitations of Viral Vectors in Gene Transfer:
The ultimate objective of gene transfer is to transmit the desired genes to subsequent generations. With virus vectors, this is not possible unless the virus is seed-transmitted. However, in case of vegetatively propagated plants, transmission of desired traits can be done e.g. potatoes. Even in these plants, there is always a risk for the transferred gene to be lost anytime. For the reasons referred above, plant biotechnologists prefer to insert the desired genes of interest into a plant chromosome.
Method # II. Direct or Vector-less DNA Transfer:
The term direct or vector less transfer of DNA is used when the foreign DNA is directly introduced into the plant genome. Direct DNA transfer methods rely on the delivery of naked DNA into the plant cells. This is in contrast to the Agrobacterium or vector-mediated DNA transfer which may be regarded as indirect methods. Majority of the direct DNA transfer methods are simple and effective. And in fact, several transgenic plants have been developed by this approach.
Limitations of direct DNA transfer:
The major disadvantage of direct gene transfer is that the frequency of transgene rearrangements is high. This results in higher transgene copy number, and high frequencies of gene silencing.
Types of direct DNA transfer:
The direct DNA transfer can be broadly divided into three categories.
1. Physical gene transfer methods—electro- portion, particle bombardment, microinjection, liposome fusion, silicon carbide fibres.
2. Chemical gene transfer methods—Polyethylene glycol (PEG)-mediated, diethyl amino ethyl (DEAE) dextran-mediated, calcium phosphate precipitation.
3. DNA imbibition by cells/tissues/organs.
The salient features of the different methods for direct DNA transfer are given in Table 49.1 .
(A) Physical Gene Transfer Methods:
An overview of the general scheme for the production of transgenic plants by employing physical transfer methods is depicted in Fig. 49.9. Some details of the different techniques are described.
1. Electroporation:
Electroporation basically involves the use of high field strength electrical impulses to reversibly permeabilize the cell membranes for the uptake of DNA. This technique can be used for the delivery of DNA into intact plant cells and protoplasts.
The plant material is incubated in a buffer solution containing the desired foreign/target DNA, and subjected to high voltage electrical impulses. This results in the formation of pores in the plasma membrane through which DNA enters and gets integrated into the host cell genome.
In the early years, only protoplasts were used for gene transfer by electroporation. Now a days, intact cells, callus cultures and immature embryos can be used with suitable pre- and post-electroporation treatments. Electroporation has been successfully used for the production of transgenic plants of many cereals e.g. rice, wheat, maize.
Advantages of electroporation:
i. This technique is simple, convenient and rapid, besides being cost-effective.
ii. The transformed cells are at the same physiological state after electroporation.
iii. Efficiency of transformation can be improved by optimising the electrical field strength, and addition of spermidine.
Limitations of electroporation:
i. Under normal conditions, the amount of DNA delivered into plant cells is very low.
ii. Efficiency of electroporation is highly variable depending on the plant material and the treatment conditions.
iii. Regeneration of plants is not very easy, particularly when protoplasts are used.
2. Particle Bombardment (Biolistics):
Particle (or micro projectile) bombardment is the most effective method for gene transfer, and creation of transgenic plants. This method is versatile due to the fact that it can be successfully used for the DNA transfer in mammalian cells and microorganisms.
The micro projectile bombardment method was initially named as biolistics by its inventor Sanford (1988). Biolistics is a combination of biological and ballistics. There are other names for this technique- particle gun, gene gun, bio blaster. A diagrammatic representation of micro projectile bombardment system for the transfer of genes in plants is depicted in Fig. 49.10, and briefly described below.
Micro carriers (micro projectiles), the tungsten or gold particles coated with DNA, are carried by macro carriers (macro projectiles). These macro-carriers are inserted into the apparatus and pushed downward by rupturing the disc.
The stopping plate does not permit the movement of macro carrier while the micro carriers (with DNA) are propelled at a high speed into the plant material. Here the DNA segments are released which enter the plant cells and integrate with the genome.
Plant material used in bombardment:
Two types of plant tissue are commonly used for particle bombardment:
1. Primary explants which can be subjected to bombardment that are subsequently induced to become embryo genic and regenerate.
2. Proliferating embryonic tissues that can be bombarded in cultures and then allowed to proliferate and regenerate.
In order to protect plant tissues from being damaged by bombardment, cultures are maintained on high osmoticum media or subjected to limited plasmolysis.
Transgene integration in bombardment:
It is believed (based on the gene transfer in rice by biolistics) that the gene transfer in particle bombardment is a two stage process.
1. In the pre-integration phase, the vector DNA molecules are spliced together. This results in fragments carrying multiple gene copies.
2. Integrative phase is characterized by the insertion of gene copies into the host plant genome.
The integrative phase facilitates further transgene integration which may occur at the same point or a point close to it. The net result is that particle bombardment is frequently associated with high copy number at a single locus. This type of single locus may be beneficial for regeneration of plants.
The success of bombardment:
The particle bombardment technique was first introduced in 1987. It has been successfully used for the transformation of many cereals, e.g. rice, wheat, maize. In fact, the first commercial genetically modified (CM) crops such as maize containing Bt-toxin gene were developed by this approach.
A selected list of the transgenic plants (developed by biolistics) along with the sources of the plant materials used is given in Table 49.2.
Factors affecting bombardment:
Several attempts are made to study the various factors, and optimize the system of particle bombardment for its most efficient use. Some of the important parameters are described.
Nature of micro particles:
Inert metals such as tungsten, gold and platinum are used as micro particles to carry DNA. These particles with relatively higher mass will have a better chance to move fast when bombarded and penetrate the tissues.
Nature of tissues/cells:
The target cells that are capable of undergoing division are suitable for transformation. Some more details on the choice of plant material used in bombardment are already given.
Amount of DNA:
The transformation may be low when too little DNA is used. On the other hand, too much DNA may result is high copy number and rearrangement of transgenes. Therefore, the quantity of DNA used should be balanced. Recently, some workers have started using the chemical aminosiloxane to coat the micro particles with low quantities of DNA adequate enough to achieve high efficiency of transformation.
Environmental parameters:
Many environmental variables are known to influence particle bombardment. These factors (temperature, humidity, photoperiod etc.) influence the physiology of the plant material, and consequently the gene transfer. It is also observed that some explants, after bombardment may require special regimes of light, humidity, temperature etc.
The technology of particle bombardment has been improved in recent years, particularly with regard to the use of equipment. A commercially produced particle bombardment apparatus namely PDS-1000/HC is widely used these days.
Advantages of particle bombardment:
i. Gene transfer can be efficiently done in organized tissues.
ii. Different species of plants can be used to develop transgenic plants.
Limitations of particle bombardment:
i. The major complication is the production of high transgene copy number. This may result in instability of transgene expression due to gene silencing.
ii. The target tissue may often get damaged due to lack of control of bombardment velocity.
iii. Sometimes, undesirable chimeric plants may be regenerated.
3. Microinjection:
Microinjection is a direct physical method involving the mechanical insertion of the desirable DNA into a target cell. The target cell may be the one identified from intact cells, protoplasts, callus, embryos, meristems etc. Microinjection is used for the transfer of cellular organelles and for the manipulation of chromosomes.
The technique of microinjection involves the transfer of the gene through a micropipette (0.5-10.0 pm tip) into the cytoplasm/nucleus of a plant cell or protoplast. While the gene transfer is done, the recipient cells are kept immobilized in agarose embedding, and held by a suction holding pipette (Fig. 49.11).
As the process of microinjection is complete, the transformed cell is cultured and grown to develop into a transgenic plant. In fact, transgenic tobacco and Brassica napus have been developed by this approach. The major limitations of microinjection are that it is slow, expensive, and has to be performed by trained and skilled personnel.
4. Liposome-Mediated Transformation:
Liposomes are artificially created lipid vesicles containing a phospholipid membrane. They are successfully used in mammalian cells for the delivery of proteins, drugs etc. Liposomes carrying genes can be employed to fuse with protoplasts and transfer the genes.
The efficiency of transformation increases when the process is carried out in conjunction with polyethylene glycol (PEG). Liposome-mediated transformation involves adhesion of liposomes to the protoplast surface, its fusion at the site of attachment and release of plasmids inside the cell (Fig. 49.12).
Advantages of liposome fusion:
i. Being present in an encapsulated form of liposomes, DNA is protected from environmental insults and damage.
ii. DNA is stable and can be stored for some time in liposomes prior to transfer.
iii. Applicable to a wide range of plant cells.
iv. There is good reproducibility in the technique.
Limitations of liposome fusion:
The major problem with liposome-mediated transformation is the difficulty associated with the regeneration of plants from transformed protoplasts.
5. Silicon Carbide Fibre-Mediated Transformation:
The silicon carbide fibres (SCF) are about 0.3-0.6 pm in diameter and 10-100 pm in length. These fibres are capable of penetrating the cell wall and plasma membrane, and thus can deliver DNA into the cells. The DNA coated silicon carbide fibres are vortexed with ‘plant material (suspension culture, calluses). During the mixing, DNA adhering to the fibres enters the cells and gets stably integrated with the host genome. The silicon carbide fibres with the trade name Whiskers are available in the market.
Advantages of SCF-mediated transformation:
i. Direct delivery of DNA into intact walled cells. This avoids the protoplast isolation.
ii. Procedure is simple and does not involve costly equipment.
Disadvantages of SCF-mediated transformation:
i. Silicon carbide fibres are carcinogenic and therefore have to be carefully handled.
ii. The embryonic plant cells are hard and compact and are resistant to SCF penetration.
In recent years, some improvements have been made in SCF-mediated transformation. This has helped in the transformation of rice, wheat, maize and barley by using this technique.
(B) Chemical Gene Transfer Methods:
1. Polyethylene glycol (PEG)-mediated transfer:
Polyethylene glycol (PEG), in the presence of divalent cations (using Ca2+), destabilizes the plasma membrane of protoplasts and renders it permeable to naked DNA. In this way, the DNA enters nucleus of the protoplasts and gets integrated with the genome.
The procedure involves the isolation of protoplasts and their suspension, addition of plasmid DNA, followed by a slow addition of 40% PEG-4000 (w/v) dissolved in mannitol and calcium nitrate solution. As this mixture is incubated, protoplasts get transformed.
Advantages of PEG-mediated transformation:
i. A large number of protoplasts can be simultaneously transformed.
ii. This technique can be successfully used for a wide range of plant species.
Limitations of PEG-mediated transformation:
i. The DNA is susceptible for degradation and rearrangement.
ii. Random integration of foreign DNA into genome may result in undesirable traits.
iii. Regeneration of plants from transformed protoplasts is a difficult task.
2. Deae Dextran-Mediated transfer:
The desirable DNA can be complexed with a high molecular weight polymer diethyl amino ethyl (DEAE) dextran and transferred. The major limitation of this approach is that it does not yield stable trans-formants.
Calcium Phosphate Co- Precipitation-Mediated Transfer:
The DNA is allowed to mix with calcium chloride solution and isotonic phosphate buffer to form DNA-calcium phosphate precipitate. When the actively dividing cells in culture are exposed to this precipitate for several hours, the cells get transformed. The success of this method is dependent on the high concentration of DNA and the protection of the complex precipitate. Addition of dimethyl sulfoxide (DMSO) increases the efficiency of transformation.
Dna Imbibition By Cells/Tissues:
Some workers have seriously tried to transform cells by incubating cell suspensions, tissues, embryos and even seeds with DNA. The belief is that the DNA gets imbibed, and the cells get transformed. DNA imbibition approach has met with little or no success.