Let us make an in-depth study of Embryo. After reading this article you will learn about: 1. Meaning of Embryo 2. Development of Embryo in Dicots 3. Development of Embryo in Monocots 4. Incompatibility 5. Special Modes of Reproduction.
Contents
Meaning of Embryo:
After fertilization, the fertilized egg is called zygote or oospore which develops into an embryo. The oospore before it actually enters into the process undergoes a period of rest which may vary from few hours to few months. Generally the zygote (oospore) divides immediately after the first division of the primary endosperm nucleus.
Unlike gymnosperms where the early stages of the development show free nuclear divisions the first division of zygote is always followed by wall-formation resulting in a two-celled pro-embryo. Practically there are no fundamental differences in the early stages of the development of the embryos of monocots and dicots.
But in late stages, there is a marked difference between the embryos of dicotyledonous and monocotyledonous plants, hence their embryogenesis has been considered here separately.
Development of Embryo in Dicots:
According to Soueges, the mode of origin of the four-celled pro-embryo and the contribution made by each of these cells makes the base for the classification of the embryonal type. However, Schnarf (1929), Johansen (1945) and Maheshwari (1950) have recognized five main types of embryos in dicotyledons.
They are as follows:
I. The terminal cell of the two-celled pro-embryo divides by longitudinal wall.
(i) Crucifer type:
Basal cell plays little or no role in the development of the embryo.
(ii) Asterad type:
Basal and terminal cells play an important role in the development of the embryo.
II. The terminal cell of the two-celled proembryo divides by a transverse wall, Basal cell plays a little or no role in the development of the embryo.
III. Solanad type:
Basal cell usually forms a suspensor of two or more cells.
IV. Caryophyllod type:
Basal cell does divide further.
V. Chenopodiad type:
Both basal and terminal cells take part in the development of the embryo.
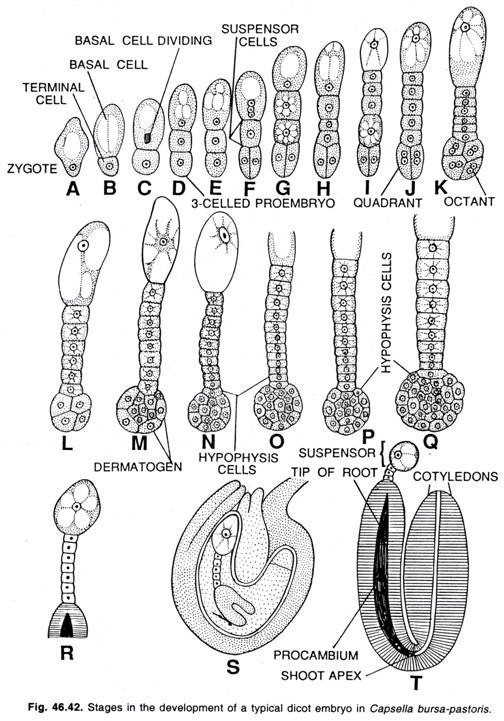
Here citing the example of Capsella bursa-pastoris (Shepherd’s purse), the detailed study of Crucifer type of the development of the embryo has been given.
Development of dicot embryo in Capsella bursa-pastoris (Crucifer type):
For the first time Hanstein (1870) worked out the details of the development of embryo in Capsella bursa- pastoris, a member of Crucifeae.
The oospore divides transversely forming two cells, a terminal cell and basal cell. The cell towards the micropylar end of the embryo sac is the suspensor cell (i.e., basal cell) and the other one makes to the embryo .cell (i.e., terminal cell). The terminal cell by subsequent divisions gives rise to the embryo while the basal cell contributes the formation of suspensor.
The terminal cell divides by a vertical division forming a 4-celled 1-shaped embryo. In certain plants the basal cell also forms the hypocotyl (i.e., the root end of the embryo) in addition of suspensor. The terminal cells of the four-celled pro-embryo divide vertically at right angle to the first vertical wall forming four cells. Now each of the four cells divides transversely forming the octant stage (8-celled) of the embryo.
The four cells next to the suspensor are termed the hypo-basal or posterior octants while the remaining four cells make the epibasal or anterior octants. The epibasal octants give rise to plumule and the cotyledons, whereas the hybobasal octants give rise to the hypocotyl with the exception of its tip. Now all the eight cells of the octant divide periclinally forming outer and inner cells.
The outer cells divide further by anticlinal division forming a peripheral layer of epidermal cells, the dermatogen. The inner cells divide by longitudinal and transverse divisions forming periblem beneath the dermatogen and plerome in the central region. The cells of periblem give rise to the cortex while that of plerome form the stele.
At the time of the development of the octant stage of embryo the two basal cells divide transversely forming a 6-10 celled filament, the suspensor which attains its maximum development by the time embryo attains globular stage. The suspensor pushes the embryo cells down into the endosperm.
The distal cell of the suspensor is much larger than the other cells and acts as a haustorium. The lowermost cell of the suspensor is known as hypophysis. By further divisions, the hypophysis gives rise to the embryonic root and root cap.
With the continuous growth, the embryo becomes heart-shaped which is made up of two primordia of cotyledons. The mature embryo consists of a short axis and two cotyledons. Each cotyledon appears on either side of the hypocotyl. In most of dicotyledons, the general course of embryogenesis is followed as seen in Capsella bursa-pastoris.
Development of Embryo in Monocots:
There is no essential difference between the monocotyledons and the dicotyledons regarding the early cell divisions of the proembryo, but the mature embryos are quite different in two groups. Here the embryogeny of Sagittaria sagittifolia has been given as one of the examples.
The zygote divides transversely forming the terminal cell and the basal cell. The basal cell, which is the larger and lies towards the micropylar end, does not divide again but becomes transformed directly into a large vesicular cell. The terminal cell divides transversely forming the two cells. of these, the lower cell divides vertically forming a pair of juxtaposed cells, and the middle cell divides transversely into two cells.
In the next stage, the two cells once again divide vertically forming quadrants. The cell next to the quadrants also divides vertically and the cell next to the upper vesicular divides several times transversely. The quadrants now divide transversely forming the octants, the eight cells being arranged in two tiers of four cells each. With the result of periclinal division, the dermatogen is formed.
Later the periblem and plerome are also differentiated. All these regions, formed from the octants develop into a single terminal cotyledon afterwards. The lowermost cell L of the three-celled suspensor divides vertically to form the plumule or stem tip. The cells R form radicle. The upper 3-6 cells contribute to the formation of suspensor.
Incompatibility of Embryo:
In nature, the stigma receives a variety of air-borne or insect-carried pollen but not all pollen that reach the stigma succeed in effecting fertilisation. The pistil allows the pollen of only the right mating type to function normally, others are discarded.
Thus, incompatibility is the inability of certain gametes, even from genetically similar plant species, to fuse with each other. If a pistil carrying functional female gamete fails to set seed following pollination with viable and fertile pollen, capable of bringing about fertilisation in another pistil, the two are said to be incompatible.
Incompatibility is also called intraspecifie incompatibility, self-sterility or self- incompatibility. The phenomenon of self-incompatibility is generally due to the prevention of some physiological or morphological mechanisms. This involves many complex mechanisms associated with interactions of pollen and stigmatic tissues.
Self-Incompatibility:
A large number of flowering plants are successfully fertilised only by the pollen of other plants and not by their own. Such flowering plants are called out-breeders.
In nature different floral adaptations, such as dichogamy, herkogamy and unisexuality have evolved to prevent self- pollination but the most widespread and effective natural device in such plants is self-incompatibility, which refers to the inability of a plant producing functional male and female gametes to set seeds when self-pollinated.
Depending on the origin of factors determining the mating types on the pollen side, two categories of self-incompatibility have been recognised:
(i) Sporophytic Incompatibility:
Where the incompatibility is due to the genotype of the sporophytic stigmatic tissues, e.g., in Asteraceae, Brassicaceae, etc.
(ii) Gametophytic Incompatibility:
Where the incompatibility is due to the genotype of pollen, e.g., in Poaceae, Liliaceae, Solanaceae, etc. This may be due to prevention of pollen germination, retardation of growth, deorientation of pollen tube, or even failure of nuclear fusion. It is controlled by genes with multiple alleles (S-allele). Usually, it develops with the maturation of stigma.
Biological Significance of Incompatibility:
In nature a balanced breeding of the plants is regulated by incompatibility. Extensive selfing of the plants leads to highly homozygous individuals which have a very low survival value. To overcome this, nature has imposed self- incompatibility.
Despite the natural value, incompatibility may turn out to be a serious hurdle in a plant improvement programme. For example, before the introduction of another culture technique selfing was one of the chief methods to obtain homozygous individuals.
For several plants where anther culture has failed to yield haploids, self-pollination continues to be an important approach to achieve this. In this context, self-incompatibility is a serious problem.
Special Modes of Reproduction:
The vegetative and sexual modes of reproduction are generally normal and occur in nature. There are cases of special modes of reproduction where propagation takes place without the act of fertilisation.
Some special nodes are mentioned here:
Apomixis:
According to Winkler (1908, 1934), the substitution for sexual reproduction of an asexual process which does not involve any nuclear fusion. The term apomixis (apo = without; mixis = mingling) is a general one, and covers all types of asexual reproduction which tend to replace, or to act as substitute for the sexual method.
This phenomenon covers all the non-sexual methods of reproduction in which the reproducing spores are developed without fusion, whereas the normal sexual cycle (amphimixis) involves two important processes: (a) meiosis, that transforms a diploid (2n) sporophytic cell into four haploid (n) gametophytic cells, and (b) fertilization, where two haploid gametes of opposite sex fuse and re-establish the diploid sporophytic generation.
Thus, in a sexual cycle, a diploid (2n) or sporophytic generation alternates with the haploid (n) or gametophytic generation. In angiosperms, the gametophytic generations are very short and are represented by embryo sac on the female side and microspore or pollen grain on the male side. The remaining part of the life-cycle represents the sporophytic generation.
The plants where the usual sexual reproduction has been completely replaced by a type of asexual reproduction are called apomictic, and the phenomenon, apomixis.
There are two main categories of apomixis, i.e.:
1. Agamospermy and
2. Vegetative reproduction.
Agamospermy:
In this category of apomixis the plants have retained seed as the agent of propagation but the embryo is formed by some process in which normal meiosis and syngamy have been eliminated. This phenomenon is known as agamospermy.
There are three different types of agamospermy:
1. Adventive embryony:
In this type of agamospermy, embryos arise directly from the diploid sporophytic cells either of nucellus or integuments. The sexual embryo sac develops in normal way and the zygotic embryo either degenerates or competes with the apomictic embryos.
2. Diplospory:
In this type of agamospermy, a diploid embryo sac is formed from a megaspore mother cell, without a regular meiotic division (e.g., in Aerva tomentosa). In this type an archesporium differentiates, but the megaspore mother cell develops into an unreduced embryo sac. The embryo is formed by the unfertilized egg, i.e., parthenogenesis or some other cell of the embryo sac, i.e., apogamety.
3. Apospory:
This phenomenon was reported in angiosperms for the first time by Rosenberg (1907). Here a somatic cell in the nucellus directly forms an unreduced embryo sac, and the diploid egg parthenogenetically develops into embryo. Here the megaspores gradually degenerate and the aposporic embryo sac may develop from a functional megaspore mother cell.
However, for the sake of convenience Dr. P. Maheshwari (1950) has sub-divided apomixis into three types, i.e.,
(i) Nonrecurrent apomixis,
(ii) recurrent apomixis, and
(iii) Adventive embryony.
i. Non-recurrent apomixes:
In this type the megaspore mother cell undergoes the usual meiotic divisions and a haploid embryo sac is formed. Here the embryo arises either from the egg (i.e., haploid parthenogenesis) or from some other cell of the gametophyte (i.e., haploid apogamy).
The plants produced by this method are, haploid and generally sterile and do not reproduce sexually any more. This type of apomixis has been seen in several species such as Solarium nigrum, Lilium, Bergenia, Erythraea centaurium. Orchis maculata, Nicotiana tabacum, etc.
ii. Recurrent apomixes:
In this type, the embryo sac generally arises either from an archesporial cell (i.e., generative apospory) or from some other part of the nucellus (i.e., somatic apospory). Here all the nuclei of the embryo sac are diploid, and there is no meiotic division.
The embryo arises either from the egg (diploid parthenogenesis) or from some other cell of the gametophyte (diploid apogamy). Generative apospory has been observed in Eupatorium glandulosum, Parthenium argentatum, etc. Somatic apospory has been reported in Hieracium excelens, H. flagellare and H. aurantiacum.
iii. Adventive embryony:
This type of apomixis is also known as sporophytic budding. Here, the developed embryo sacs may be haploid or diploid, but the embryos do not arise from the cells of nucellus or the integument. There is no alternation of generations, because the diploid tissues of the present sporophyte directly give rise to the new embryo.
Adventive embryony has been frequently reported in Citrus, Euphorbia dulcis, Capparis frondosa, Mangifera indica and Hiptage madablota.
Significance of apomixes:
The most important apomictic crop plants are citrus, mango, mangos-teen and black berries. As a reproductive system, it offers the possibility of the indefinite propagation of specially favourable biotypes, which may be highly heterozygous or sexually sterile.
In obligate apomixis, this advantage is served at the expense of the long term evolutionary flexibility which is the gift of sexuality. However, in facultative apomixis, where sexual and apomictic members co-exist, the phenomenon is of special significance.
Vegetative Reproduction:
In this type of apomixis, the new individual arises from a group of undifferentiated or differentiated cells, where neither embryo nor seed are produced. This type of reproduction takes place by means of bulbs, bulbils, tubers, runners, suckers, etc. Such propagules are formed by the sporophyte only.
Gustafsson (1946) has distinguished three types of vegetative reproduction in angiosperms:
1. In this type, the propagules are formed outside the floral regions; though the sex organs are formed, yet no fertilization or seed setting takes place, e.g., in Agave americana and Elodea canadensis.
2. In this type, the propagules are formed outside the floral regions, and the plants are sexually sterile, as found in Fritillaria imperialis and Lilium bulbiferum.
3. In this type, the propagules are formed on the floral branches either in addition to the flowers or in place of them. This phenomenon is commonly known as vivipary.
This term is also used for those plants (e.g., mangrove vegetation), in which sexually formed seeds germinate on the mother plant. Here the term vivipary will be used as vegetative vivipary. The vegetative vivipary is commonly found in grasses, e.g., Festuca, Poa, and in Allium.
The vegetative vivipary is actually an adaptation for the multiplication of a genotype under a set of environmental conditions which prevent or check to some extent the opportunity for normal pollination.
However, most of the vegetative viviparous races have not lost the capacity for flowering and normal seed setting, e.g., a grass, Deschampsia caespitosa is sexually reproducing in Sweden, while growing by vegetative vivipary in California.
Apospory:
The apospory (apo = without) is the development of the gametophyte from a cell of the sporophyte without the intervention of a spore. In angiosperms, this phenomenon was for the first time reported by Rosenberg (1907) in Hieracium spp.
Here the megaspore mother cell undergoes the usual meiotic divisions and forms a tetrad. In the angiosperms it is sometimes seen that an embryo may be formed from the diploid cells of the nucellus, as in Citrus, Mangifera, Opuntia, etc., or even from those of integument, as in Allium cepa.
The embryo thus formed is pushed into the embryo sac during the course of its development. Since the nucellus or the integument belongs to the sporophyte, the production of the embryo from the tissue of the sporophyte without the intervention of the spore is a case of apospory.
In the aposporic members of the Asteraceae, only one nucellar cell acts as the mother cell and gives rise to a normal 8- nucleate embryo sac, while in the grasses, more than one aposporic embryo sac may develop in the same nucellus, and the organization of mature embryo sac is 4-nucleate.
Parthenogenesis:
The development of the zygote from the egg-cell without the act of fertilization, or in other words, formation of embryo from an unfertilized egg is called parthenogenesis. In some species of angiosperms, the embryo develops parthenogenetically.
In such cases the embryo may develop from haploid egg-cell or diploid egg-cell. In apomicts of Asteraceae and Rubiaceae, the development of embryo is independent of the pollination stimulus.
However, in many other apomicts, the embryo develops only after pollination, and the phenomenon is known as pseudogamy, e.g., many apomictic grasses.
According to Heslop-Harrison (1972) there are three roles of pollination in pseudogamy:
(i) To activate the growth of ovary and ovule,
(ii) To supply the male nucleus for the development of the endosperm, and
(iii) To stimulate the parthenogenesis.
In most of grass apomicts, the egg may divide parthenogenetically but the pro-embryo ceases to grow unless and until the endosperm develops. Here, the development of endosperm occurs only after the fusion of the male- nucleus with the polars, and therefore, a mature apomictic embryo in these plants is formed only after pollination.
Significance of parthenogenesis:
The role of parthenogenesis in nature is severely limited; the zygogenesis and other methods of reproduction are predominant. The reason is thought to be that parthenogenetic species are not adaptable enough. In ameiotic parthenogenesis, genetic variability is practically nil. In meiotic parthenogenesis, individuals tend to become homozygous, and cause the accompanying disadvantage of this condition.
The advantages of this phenomenon are several fold. In meiotic parthenogenesis which applies to homozygous genotypes, advantageous combinations of genes are maintained instead of dispersed by meiosis as in zygogenetic species.
Well adapted forms thus spread rapidly, as long as the environmental conditions remain unchanged. As Peacock (1961) reports, the existence of hardly triploid organism becomes possible in ameiotic parthenogenesis.
Parthenocarpy:
In certain cases of angiosperms the ovary normally develops into a fruit without pollination and fertilization. This type of free development of fruit is known as parthenocarpy. Such fruits (parthenocarpic fruits) are always seedless. Sometimes the fruit formation may be induced by artificial pollination by foreign pollen from another species, but without subsequent fertilization.
The parthenocarpy may also be induced by the spraying of growth promoting substances, such as naphthalene acetic acid NAA. This is called induced parthenocarpy. The examples of parthenocarpy are commonly found in banana, papaya, pineapple, guava, grapes, apple, Thalictrum, Alchemilla, etc.
In modern days, a lot of work has been done to produce seedless fruit on seeded varieties by controlling pollination and applying certain chemical substances to the pistil. Thimann (1934) suggested that many pollen grains possess considerable quantities of growth substances.
Gustaffson (1938) used several growth substances, such as IAA, IBA, α-NAA and phenylacetic acid mixed in the lanolin paste of about 0.5 to 1 per cent strength and smeared on the stigma to produce parthenocarpic fruits. Among the species where the fruits can be set by auxins are tomato, tobacco, pepper, figs and blackberry.
Here, the fruits which have been set by treating un-pollinated flowers are seedless. Nitsch and other, workers used α-NAA in aqueous form to induce parthenocarpy by atomizer. Crane (1964) induced parthenocarpy in tomatoes, apples and pears by gibberellins. Cytokinins have also been used in the induction of parthenocarpy in certain fruits.
In addition to induced parthenocarpy, the natural parthenocarpy may occur in certain species. Many horticultural varieties of bananas, pineapples, cucumber, tomatoes and figs exist, where seedless fruits are normally produced without the need for any exogenous hormone.
In some species, the fruits are formed without pollination while in others, pollination is necessary but fertilization does not occur; in still others fertilization occurs but the embryos abort before the fruits mature.
It is not understood clearly how the growth of these parthenocarpic fruits is controlled, but it is thought that in some cases the maternal tissues, such as the placentae may produce auxin in the absence of normal embryos. It has been noticed that the ovaries of unopened flowers of parthenocarpic varieties of orange and Vitis vinifera (grape) possess a higher auxin content than those of normal seeded varieties.
Polyembryony:
The occurrence of more than one embryo in the seed is known as polyembryony. Polyembryony is quite common among conifers (gymnosperms), but many species both of dicots and monocots (angiosperms) exhibit this phenomenon.
Usually there are two main types of polyembryony, i.e.:
1. True polyembryony and
2. False polyembryony.
1. True Polyembryony:
The true polyembryony may be subdivided into two types:
(i) Cleavage polyembryony, where the embryos arise within an embryo sac, either by a cleavage of the egg, or from the synergids, antipodals or endosperm;
(ii) Adventive polyembryony, where the embryos arise from the tissues living outside the embryo sac, i.e., the cells of the nucellus or the integuments, but generally they come to lie within the embryo sac.
2. False Polyembryony:
Sometimes the polyembryony occurs due to the presence of multiple embryo sacs within the ovule.
They may arise from:
(a) The derivatives of the same megaspore mother cell;
(b) From two or more megaspore mother cells, or
(c) From nucellus cells (i.e., apospory).
Importance of Polyembryony:
This phenomenon plays an important role in plant breeding and horticulture. Nucellar adventive polyembryony is of great value in horticulture, where the nucellar seedlings of Citrus have been proved to be better clones of orchard stock than cuttings.
On the other hand, the nucellar embryos are supposed to be free from disease, and the nucellar seedlings rejuvenate the vigour that is generally lost after continued cutting propagation. The adventive polyembryony is much useful in the propagation of the fruit trees, such as Citrus and Mango. The application of adventive embryos is also important for providing genetically uniform seedlings in fruit trees.
The haploids can be used for the development of homozygous diploid, which are of much value. Due to the practical value of haploids in plant breeding, the methods have been recognized for the artificial production of these embryos from the eggs or synergids.