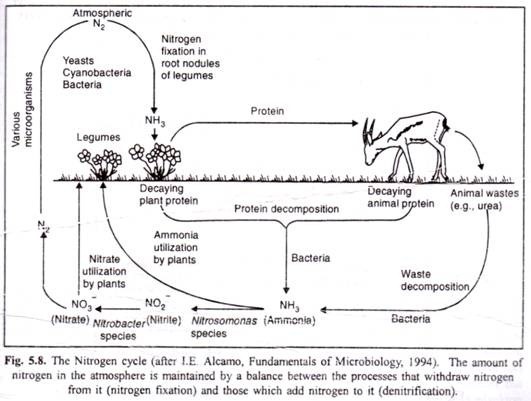
Nitrogen being 79 per cent of the atmosphere, the atmospheric phase is predominant in the global nitrogen cycle. It is required by organisms in the synthesis of proteins, nucleic acids, and other nitrogenous compounds.
Atmospheric nitrogen serves as the ultimate source. But aerial nitrogen, the most abundant component of air, is rather inert chemically and actually cannot be used as such by the majority of organisms.
It must first be “fixed” by specialized organisms or by industrial processes.
From an ecological perspective, the nitrogen cycle consists of the following stages:
(i) Ammonification
(ii) Nitrification,
(iii) Nitrogen uptake by plants,
(iv) Fixation of Nitrogen, and
(v) Denitrification
Ammonification:
Ammonification is a process in which the organic nitrogen of plants and animals after their death is converted to ammonium ions (NH4) by the action of saprotrophic fungi and bacteria. The saprotrophs use the ammonia (NH3) to synthesize their own proteins and other nitrogen-containing organic compounds.
Nitrification:
Ammonium ions added to the soil by ammonification, are soon oxidized by a process known as nitrification. It takes place in two stages. In the first stage, ammonium (NH4+) is converted to nitrite (NO2-). This reaction involves the addition of oxygen to ammonia, giving rise to hydroxylamine (NH2OH), which is further oxidized to nitrite. This reaction is completed by the bacteria such as Nitrosomonas, Nitrosospira, Nitrosolobus and Nitrosococcus (Hamilton, 1988).
The second stage of nitrification involves the oxidation of nitrite (NO2) to nitrate (NO3) by bacteria of the genera Nitrobacter, Nitrospira and Nitrococcus. The reaction proceeds by the addition of water followed by the removal of hydrogen (Hamilton, 1988). The bacteria responsible for these reactions occur in the same soil. The association between Nitwsomonus and Nitrobacter has been described as one of commensalism (Gooday, 1988).
Nitrogen uptake by Plants:
Nitrate (NO3–) formed in the process of nitrification is used by most plants as a mineral metabolite and may be converted by them into amino groups and other nitrogen- containing compounds. Nitrates are also added to the soil through rock dissolution and combination of atmospheric nitrogen with oxygen by lightning (nitrates so formed reach the soil by rain). However, many plants also absorb ammonium from the soil.
Fixation of Nitrogen:
The reduction of atmospheric nitrogen (N2) to the ammonium ion (NH+4) is called nitrogen fixation. This process can only be carried out by certain species of bacteria and cyanobacteria (Postgate, 1988). Some of these bacteria are free-living, occurring in soil or water (saprophytic bacteria like Azotobacter and Clostridium ; others exist in symbiotic relationship with plants of the Family Legxuninosae, e.g., nodule bacteria Rhizobium leguminosarum).
Species of the genus Rhizobium occur in the soil until they infect a damaged epidermal cell or root hair. The plant responds to this infection by producing root nodules, about 1 to 3 mm in diameter. These root nodules contain leg hemoglobin, which functioning like hemoglobin, transports oxygen.
As these bacteria are aerobic, some oxygen is required for the bacteria to survive, but too much oxygen inactivates the enzyme nitrogenase that catalyses nitrogen fixation. The nitrogen molecule (N2) is very stable and 16 molecules of ATP are needed for each molecule of nitrogen that is fixed. In the soil microorganism Klebsiella pneumoniae a total of 17 genes, called ‘nif’ genes are known to be responsible in nitrogen fixation.
Researches in biotechnology are now attempting transfer of ‘nif’ genes from prokaryotes to crop plants so that yield of crops like rice and wheat may be increased. However, scientists have not yet succeeded in this attempt.
It is a process in which the nitrate ion (NO3) is reduced to nitrogen dioxide (NO2), di-nitrogen oxide (N2O), nitrogen monoxide (NO) or nitrogen (N2) by certain soil bacteria like Pseudomonas denitrificans. Thus, nitrogen is liberated into the atmosphere. Plants also lose small amounts of nitrogen to the atmosphere as gaseous ammonia, N2O, NO2 and NO especially when well fertilized with nitrogen (Wetselaar and Farquhar, 1980).
Thus, nitrogen cycle (Fig. 5.8) depends upon at least four different kinds of bacteria known as the decay causers, the nitrifiers, the denitrifiers, and the nitrogen-fixers and there is a regular circulation of nitrogen through the air, soil, plants and animals.