In this article we will discuss about Cell:- 1. Meaning of Cell 2. Discovery of Cell 3.Tools for Viewing 4. Types 5. Dimensions 6. Functions 7. Elemental Composition.
Contents:
- Meaning of Cell
- Discovery of Cell
- Tools for Viewing the Cell
- Types of Cell
- Cell Dimensions
- Functions of Cell
- Elemental Composition of Cell
Contents
1. Meaning of Cell:
The fundamental unit of life is not an atom, ion or molecule, although these particles are present in non-living as well as in living bodies. To understand the difference between living and non-living substances a knowledge of the cell is essential.
The ions and molecules are precisely arranged in the cellular structure of an organism to provide the environment and organisation for running the life processes in order. The cell, itself, is considered to be the basic structural and functional unit of life as it is the smallest unit of all living organisms capable of carrying out all the activities necessary for life.
The word ‘cell’ is derived from Latin cella to cover i.e., it covers and encompasses the basic unit of life. It can also be said that the cell is a complete unit of metabolism because it has all the chemical and physical factors necessary for its growth and maintenance. An isolated cell is also capable of growth and differentiation in a laboratory if the proper nutrients and appropriate environments are given.
2. Discovery of Cell:
The discovery of microscope by Anton van Leeuwenhoek (1632-1723) helped the biologists to understand the basic level of organisation of living things where all structures can be reduced to a basic unit. In 1665, Robert Hooke (1635-1703) discovered that the cork, the bark of a tree, was composed of tiny compartments by using a compound microscope of his design.
He called these compartments or holes as cells. Other biologists found similar cells with full of fluid or sap in living tissue with the help of a microscope. Following this, the idea gradually cropped up on biologists that all living matter is made up of cells. In other words, the cell is the unit of life. One of the earliest proponents of this idea of cell theory was Rene’ Joachim Henri Dutrochet (1776-1847).
He published this view in 1824 but it went unnoticed. The cell theory became popular only after Matthias Jakob Schleiden (1804-1881) and Theodor Schwann (1810-1882) of Germany independently formulated it in 1847.
In 1847, T. Schwann gave the idea of tissues as:
All tissues are made up of cells in a very diversified manner but there is one principle of development. This principle is the formation of cells.
The author Schleiden formulated the cell theory as follows:
Each cell has two functions, one pertaining to its development, the other an intermediary, since it has become an integrated part of a living organism.
After a few years, in 1858, Rudolph Virchow (1821-1902) formulated the cell theory as: every living organism appears as a sum of vital units, each of which bears the complete characteristics of life. Before Schleiden and Schwann, biologists believed in Vitalism.
According to them, no single unit of an organism was alive but the properties of living things were shared by the organism as a whole. It was also thought that a primitive form of protoplasm was the germinal material of most organisms. The cell theory first showed that organisms could arise from the growth of cells.
Up to the time of Louis Pasteur (1822-1895), cells were thought to be originated through spontaneous generation and were not considered to be capable of an inheritance of their own. Pasteur was the first to show that microorganisms would grow in a culture medium if a few microbes are placed into the medium. In the end of nineteenth century, the cell theory was widely accepted.
The cell theory of Schleiden and Schwann states that:
a. All living things are composed of one or more cells;
b. New cells are formed from the pre-existing ones through divisions;
c. There are basic similarities in chemical compositions and metabolic functions of all cells;
d. The activity of an organism is the collective activities and interactions of its cellular structures, i.e., cell is the functional unit of tissues and organs.
3. Tools for Viewing the Cell:
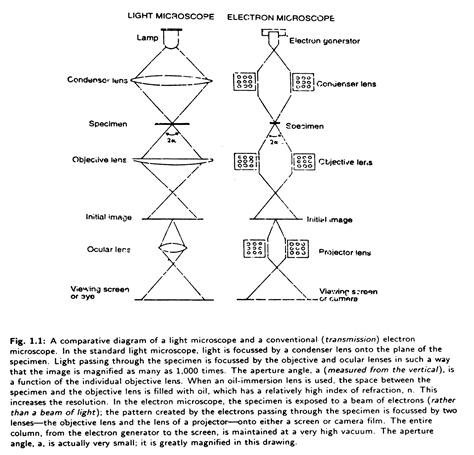
The most important tool in viewing the cell is the light microscope, which has been greatly sophisticated from the primitive one used by Robert Hooke. This microscope used by all laboratories is working on the visible light as the only source of illumination.
The modern light microscopy is based on the principle of Kohler illumination. The principle and purpose of Kohler illumination is to obtain an uniformly lighted field of view against, which the detail of the specimen can be easily recognised. The second objective is to provide maximum light to the specimen with as wide a cone of radiation in order to achieve maximum resolution of the specimen.
To apply Kohler system in modern microscopy the following principles of the method are adopted:
i. An image of the light source is placed in a position, which is not in the plane containing the specimen by putting a lens in front of the source of light.
ii. A condenser, i.e., a second lens, places the image of the first lens onto the specimen to produce a wide cone of light in illuminating the specimen.
The speciality of the Kohler illumination is the formation of two sets of planes, which are at right angles to the optical axis of the microscope. They are known as conjugated planes where the features in an earlier plane are repeated in a subsequent one.
Thus, the plane containing lamp filament or source of light is conjugated with the plane containing the iris of a condenser, which controls the aperture of the condenser.
This is again conjugated with the plane containing the back focus of the objective and is finally conjugated with the plane containing the Ramsden Disc (Fig. 1.1):
Using Kohler illumination system the observer can achieve control of stray light, i.e., unwanted light, and thus the degradation of images can be avoided. Thus the objective of light microscopy is to allow the study of fine detail in a specimen with a maximum degree of accuracy.
Another sophisticated light microscope is the Phase Contrast Microscope (Fig. 1.2) with special lenses, which has the great advantage in viewing the cell and its components in the living condition without using any stain. This is possible cine to its increased sensitivity in the detection of slight differences in the refractive index of the different components of the cell.
This microscopy has made a major improvement to the techniques available for observing living cells and is now routinely used in a wide range of work to measure different parameters of nuclei in cells. One disadvantage in phase contrast microscopy is that the image is sometimes, surrounded by a halo of bright light. This causes measurement of very small objects difficult.
The other recent development in microscopy is the Interference Contrast Microscopy which rapidly replaces the Phase Contrast one. Using modern microscopical techniques, the information about the specimen gives not only a picture of what the specimen looks like but also provides information about its physiochemical nature.
It gives an information about the refractive index, absorptive power, lack of birefringence, measurement of the difference in the optical path (o.p.d.) which again helps in measuring the concentration of protein or mass of nuclei.
Kuljinski and Pappelis (1971) from their study on epidermal cells of onion reported that the nuclei have a mass per unit area of 1 x 10-14 g/µm2 and a total nucleus weight is about 14 x 10-10 g. Actually the phase contrast microscope images are made up by the interference of waves of light whose relative phase has been changed by this microscope.
This image forms a map of the optical path differences between the specimen and the background.
The refractive index of a protein solution depends on their concentrations so the image of a phase microscope shows a map of the mass or concentrations of proteins and other components present in a cell. Again, the phase contrast system has been improved with the development of Dark-field microscopy.
Fluorescence microscopy is used to observe the minute objects in the cell and also for making differential stain in the cell. Some dyes are used for producing fluorescence, which are known as Fluorochromes or Fluorophores, such as Acridine Orange, DAPI, Quinacrine hydrochloride, Quinacrine mustard, FITC etc.
The staining through fluorochromes is based on the principle that some substances absorb light in short-wave length range which then emit light of longer wave-length giving specific colour. Thus, the emitted light is different from the absorbed light both in the intensity and wave-length.
The main components of this microscope are:
A source of illumination, i.e., a high pressure mercury vapour lamp giving a light of short wave-length; excitation filters, and barrier filters.
There are two types of illumination transmitted and incident (Figs. 1.3 and 1.4):
The incident light illumination (epifluores cense microscope) is advantageous because both fluorescence and phase contrast microscopic observations can be made in the same specimen.
The maximum use of fluorescence microscopy in Cell Biology comes from the immunofluorescence studies of Coons in 1940 who actually located antigens in cells using antibodies conjugated to fluorescent dye like FITC (Fluorescein isothiacyanate).
With the development of Immuno-cytology, the fluorescent microscope is widely used as a routine in many Cell Biology and Biotechnology laboratories. There are many applications of Fluorescence Microscopy in Cell Biology.
Mitochondria can be seen in living cells with dyes like Rhodamine 123, Rhodainine 6G and other Mito Tracker dyes.
Endoplasmic reticulum can be seen with labelled Con A, DiOC6, ER-Tracker Blue white DPX. Golgi bodies can be seen with labelled C5 and C6 ceramide, and DNA in nuclei with DAP1, Hoechst 33257, Acridine Orange, Pro-pidium iodide etc. Fluorescent labelled probes are used in the in situ hybridisation (FISH) technique.
Some of the new dyes give improved contrast and brightness. These are Texas Red Cy3, Bodipy FL, Alexa 488, GFP, Lucifer yellow etc. Of the fluorescence microscope Epifluorescence Microscopy is commonly used with illuminations by incident light.
In Epifluorescence Microscopy, the objective lens is used both for illuminating the specimen with the excitation light as well as collecting the stimulated fluorescence. The dual purpose is possible in the presence of an optical filter block consisting of an excitation filters a dichroic mirror and barrier filters.
The excitation filter is used to select the wave length of light for the excitation beam to excite the specimen on the slide which will give longer wavelength fluorescence.
This longer wavelength will only pass through the eyepieces, other lights will be prevented by the barrier filter. For chromosome study through .FISH technology the numerical aperture of the lenses is very important.
Numerical aperture is shown by n sin θ where n is the refractive index of the medium between the specimen and the lenses, θ is the half single of the cone of light collected by the lens. Generally, high numerical aperture will give high resolution in the microscope.
Polarizing Microscopy is a powerful instrument for examining cells and their components for qualitative and quantitative analysis. Structures like filaments, microtubules, matrix fibres, DNA, lipids etc. can be seen with sufficient contrast in this microscopy. Ordered structures show birefringence.
It is basically an ordinary bright field microscope fitted with a polarizer below the condenser and the second polarizing filter, called the Analyser is above the objective (Fig. 1.5). When light as a wave, vibrates in two vectors at right angles to each other and passes through a Nicol prism or through Calcite crystal the two beams sure split.
The polarizer generally passes light in a single plane and little .light is transmitted to the eyepiece after passing through Analyser. Only the changes in the polarised light by ordered structure of the cell will generate birefringence. For analytical work, a compensator is placed between the polarizer and analyser to measure the degree of birefringence.
Microtubule assays, Mitotic spindle, DNA etc. show birefringence. Recently Innoue with Hamamatsu and Olympus have produced a Centrifuge Polarizing microscope to visualize live cells and their organelles under centrifugation with high resolution.
Confocal Microscopy is now very popular as it may overcome the problem of out-of-focus plane in microscopy. In this microscopy, a specimen can be scanned with the beam of light by either stage scanning or by beam scanning systems.
Nowadays Laser scanning confocal microscopy is the most prevalent. In confocal microscopy, the out-of-focus plane fluorescence is minimised, optical sectioning can be done and, through collection of images, 3D reconstruction is done. The advantages of removing glare, looking at thicker specimens without cutting sections, and high degree of resolution has made the use of this microscopy in Cell biology in a great deal.
However, there are some disadvantages. The laser beam can cause photo bleaching and also causes cell damage due to some free radicals generated through phototoxic effect. So the two photon microscopy was invented by Denk, Stickler and Webb in 1989. Here the imaging is same as that of confocal microscopy where photo bleaching and cell damage are reduced.
In two photon microscopy, infrared wavelength high energy femta-second pulsed lasers are used. The excitation of a fluorescent dye molecule by one photon stimulates it to short lived state which then goes to excited state if a second photon hits simultaneously. The red wavelength penetrates deeper than ultraviolet, so the imaging range is extended. Thus this microscope has added advantage over other microscopy.
Cameras:
Ordinary cameras used for photomicrography are now replaced by electronic camera or Digital Camera, where a digital image of the specimen is obtained. This image consists of an array of picture elements (pixels) which measure the intensity and colour of the image in a binary coded system. This can be processed and displayed by the computer.
Then comes the use of Video camera having an image intensifier. The intensifier converts photons into an electron image and present it to the camera for commission to a video output. Again, this is replaced by solid state cameras based on charge coupled device called CCD camera. This CCD camera is now used for’ FISH technology.
It consists of a photosensitive element of silicon base, where photon induced charge can be stored in a CCD array. An image projected on to this element (array) produces different pattern of charge on each photosensitive area (pixel).
Then this is read out by the computer. The resolution of CCD camera is determined by the physical arrangement of pixels in the array. The CCD camera is generally a monochrome device but 3 types of colours like red, green and blue can be produced by using filtered detector elements.
Electron Microscope:
A great breakthrough in biology was made in 1950 when the first Transmission Electron Microscope (TEM) was discovered. It used electrons instead of visible light and magnetic field instead of glass lenses giving a resolution to 0.2 nm and magnification is about 1,000 times more than the ordinary microscope and 500,000 times more than the human eye.
With the help of this microscope, study of sub cellular structures of diameter up to 0.1-0.5 nm has thrown a new light in Cell Biology.
In 1960, the Scanning Electron Microscope (SEM) was developed. This microscope is used to observe the surfaces of the objects in detail and also the three dimensional structure of biological objects which is not possible with the ordinary microscope.
In SEM, the beam is scanned across a sample through condenser, objective and field lens and the secondary electrons given off by the surface are collected on a detector. Then these are converted to photons which are transmitted to plotomultiplier tube and are displayed on a screen through Scanning electron microscope.
Samples are generally fixed, dehydrated, dried either through critical point drying or freeze drying and are then coated with metal (gold or gold-palladium) on the surface by Sputter- coater.
Whole cells, sections, organelles can be seen with resolution at 1 nm or less. 3D images can show details on cell surface and cytoskeletal structure. Special SEMs with ultra-vacuums can give magnifications by one million with high resolution.
Another special type of SEM, i.e., Environmental SEM (ESEM) is used without high vacuum. They work in the range of 1- 20 torr. The advantage of the ESEM is to see the unfixed hydrated samples without distortion.
Cells and other tissues can be imaged in hydrated form in EM resolution without any artifacts depending on the specimen preparation methodology. Large number of analytical probes can be incorporated into SEMs making it Analytical SEMs. Thus SEM has many uses in Cell Biology which can produce 3D images of cells and other components.
4. Types of Cell:
Cell, the basic unit of all living organisms, is divided into two large groups—Prokaryotic cell and Eukaryotic cell. Bacteria and Blue-green algae are the examples of Prokaryotes and the remaining plants and animals are Eukaryotes. Prokaryotes have no definite nucleus bounded by nuclear membrane.
The nucleic acid is not associated with protein and the daughter nuclei are generally formed automatically with the help of binary fission. The eukaryotic cells have a definite nucleus containing DNA, RNA and protein with distinct nuclear membrane, discrete chromosomes and different kinds of cell organelles.
The prokaryotic cells are primitive and are found even in an anaerobic condition. These cells could not synthesise their necessary compounds needed for cell metabolism by itself. Hence, these are called Heterotrophs. But there are some autotrophic bacteria which can synthesise their organic requirements by reducing atmospheric carbon dioxide under anaerobic conditions.
The eukaryotic cells are evolved from Prokaryotes through a microbe-lacking cell wall. This wall-less microbe is called Mycoplasma (Thermo plasma acidophxlum) living in an acid environment of pH 1 to 2 at 59° C. It has some eukaryotic characteristics with the association of proteins similar to histone and nucleic acid.
This evolution of eukaryotic cells was going on simultaneously with the origin of mitochondria and plastids inside the cell due to the symbiotic association of some prokaryotes. So, the plastids and mitochondria are thought to be the descendants of prokaryotes.
i. Structure of Prokaryotic Cell:
Bacteria:
The cells of bacteria (Fig. 1.6) are smaller than eukaryotic cells. The cell-wall of bacteria is made of complex structure of polysaccharides called Peptidoglycan. The chemical components of the cell-wall in bacteria are not constant.
The differences in the chemical composition of the cell-wail have played an important role in identifying the bacteria as Gram positive or Gram negative. The Gram negative bacterium has some other cell-wall components like lipopolysaccharides and lipoprotein complexes in addition to peptidoglycan.
The cell membrane in bacteria is folded inwards to form a structure called Mesosomes. Most of the bacterial lipids and proteins are located in the mesosomes. By staining certain cells of bacteria with Feulgen reagent (a stain specific for DNA), the centrally located nuclear material is found showing that prokaryotic cells also have a nucleus-like structure (nucleoid) at the centre of the cell.
The DNA is folded back many times in the nucleoid. This single large DNA molecule is present without any association with protein. Some bacterial species have small circular DNA called Plasmids in addition to their genomic DNA.
These plasmid DNAs are responsible for coding the characters resistant to antibiotics and other toxic materials. These Eire now regarded as the most important tool in the recombinant DNA technology and genetic engineering.
Some prokaryotic cells like Cyanobacteria (Blue-Green Algae) and photosynthesizing bacteria contain plastids in their cytoplasm. Some bacterial cells have flagella which are different from that of eukaryotic cells. Large number of ribosomes are present in the cytoplasm.
Blue-Green Algae (Cyanobacteria):
The blue-green algae occur either as a single cell or large masses of single cells covered by mucilaginous sheath or as filaments where row of cells are arranged in chains. The cell-walls of the Cyanophyceae (Blue- Green Algae) are composed of a number of layers. Only the inner two layers (L1 and L2) are present in all blue-green algae.
The layer of mucilage is present outside the layer L2 and the content of mucilage varies with the environment. The cell-wall is a peptidoglycan polymer like that of bacterial cell-wall constituting up to 50% of the dry weight.
The cell structure of the Cyanophyceae is basically the same as that of Gram-negative bacteria indicating a. possible relationship between the two. The mucilage layer is fibrillar and functions in protecting the cells from drying.
The circular fibrils of DNA without any association with protein (histones) are present in the central region of the protoplasm. The amount of DNA in unicellular blue-green algae varies from 1.6 x 109 to 8.6 x 108 Daltons.
This is similar to genome size in bacteria (1.0 x 109 to 3.6 x 109 Daltons) and is larger than the genome size in mycoplasmas (0.4 x 109 to 0.5 x109 Daltons). The peripheral cytoplasm is composed mainly of thylakoids phycobilisomes and, glycogen granules.
The 703 ribosomes are dispersed throughout the blue green cell but are present in high density in the central region of the protoplasm. Flagella are absent in blue-green algae. Storage granules similar to glycogen are found in the cells of the blue-green algae.
As cells of the blue-green algae show similar ultra-structural, genetical and biochemical characteristics with the bacteria, so the blue-green algae are sometimes called Cyanobacteria.
ii. Structure of Eukaryotic Cell:
The cells of algae, fungi, protozoa, higher plants and higher animals are known as Eukaryotic cells (Fig. 1.7). These cells differ from prokaryotes in having a distinct membrane-bound nucleus and some membrane-bound cytoplasmic structures called organelles with some specialised functions.
There are also other differences that will be more clear in dealing with cell functions. Again, plant and animal cells (Fig. 1.8) differ in certain characteristics. Plant cells have a cell-wall and plastids which are not found in animal cell (Table 1.2).
The eukaryotic ceil is a complex structure with self-controlled systems of generating energy, transportation of necessary materials for its metabolic activities and also with an autolytic (self-destructing) process. The eukaryotic nucleus is bounded by two phospholipid containing membranes.
The outer membrane is continuous with the cytoplasmic membrane system called the rough endoplasmic reticulum. The inner and outer membranes are fused together at the nuclear pore regions. These nuclear pores are responsible for maintaining the nucleocytoplasmic connections between them.
In the non-dividing nucleus, one or two round structures are present—called the Nucleolus. The nucleolus is responsible for the synthesis of ribosomal RNA. The non-nucleolar region, i.e., major portion of the nucleus, is called the Nucleoplasm.
Most of the chromosomal DNA is present in the nucleoplasm. The nucleus is the active site for all cellular metabolic activities. Detailed discussion of the structure of nucleus and other organelles will be discussed later.
5. Cell Dimensions:
Cell dimension has an important role in creating a complex substructure in a little space. It maintains the cell surface area which is needed for exchange of nutrients and waste products. The ratio of surface area and cell volume varies with the size of the cells.
Smaller cells have a higher ratio than larger cells. So, the size of the cell ranges from 1-30 μm in diameter. To accommodate the uptake of more nutrients in the cell, the membrane of large cells sometimes becomes modified with the formation of large number of invaginations.
Cells of unicellular organisms like Amoeba are also sometimes modified to attain a size as great as to several millimeters. Again, some egg cells—as in hen or ostrich—are increased to a few millimeters but these may be taken as an unusual condition as these eggs contain mainly stored food.
It has been calculated that the minimum amount of proteins needed for carrying out essential metabolic functions is 10-15g of protein. This amount is the same as is found in the smallest known organisms—Mycoplasma. These cells are about 0.1 µm in diameter. The size of some cells, sub-cellular organelles, viruses and molecules is shown above.
Dimensions of different types of cells are shown in Table 1.3.:

6. Functions of Cell:
Although there are lots of difference in size and shape of various types of cells, there are basic similarities at the functional level.
The following basic functions are more or less similar:
1. All cells maintain a barrier that protects the contents of cells from the external environment. This barrier maintains the concentration of the solutes in the cell by regulating the transport of materials from in and out of the cells. Even the barriers are used to compartmentalize the cell for some specialised functions.
2. Inheritance and transmission of genetic material from one generation to another are performed in the cell through cell division. Actually, the genetic material is duplicated before cell divisions so that newly formed cells can get a full set of genetic material from the mother cell.
3. All cells carry out series of chemical reactions for the synthesis of macromolecules, trapping energy, the degradation of some unused molecules, converting food substances into sugar etc. In other words, they are performing the most important process—Metabolism to perform all the essential or cellular activities.
4. Cells can show different types of motility— from locomotion to the movement of some components of the cell.
In certain organisms, some of these functions are performed by certain specialised structures called Organelles.
i. Functioning as Barriers Due to Membranes and Cell-Walls:
Both prokaryotic and eukaryotic cells are enclosed by a thin membrane called Plasma Membrane, 8-10 nm thick. The function of the membrane in all types of cells is the same though there are some minor structural and chemical differences.
The main function of the membrane is to control the exchange of materials between the cell and its environment. According to the need of the cell, the membrane is sometimes folded or invaginated to increase the surface area for more exchange of materials.
These main cellular functions are performed for the presence of three important properties of the membrane:
a. Membranes are selectively permeable allowing certain molecules to pass in and out of the cell.
b. Membranes can selectively allow certain specific molecules against a concentration gradient maintaining a higher concentration inside the cell than outside, or vice versa. This process requires some energy and this is known as Active Transport.
c. Excess amount present in the cell are thrown out with the formation of vesicle. These vesicles are ‘pinched off’ and released from the cell containing various substances. This type of movement—out of the cell—is called Exocytosis. Endocytosis includes the process of movement of contents within the cell.
d. Besides these, the membranes also contain some proteins and enzymes to perform certain specific metabolic activities.
e. The membrane also maintains the physical integrity of the cell.
Although membranes are present in the outer surface of the cell, they are also present in the cytoplasmic organelles. This compartmentalization of the cell, i.e., the formation of different organelles for certain specific functions, is more pronounced in eukaryotic cells.
Very few membrane organisations are found in prokaryotic cells like:
a. Chromatophores or photosynthetic lamellae are parallel shoots of membranes taking part in photosynthesis;
b. Chon-droids are also sheets of membranes having a role in respiration;
c. Mesosomes of bacteria are also the invaginations of membranes, the specific function of which is not very clear.
It may take part in energy metabolism and during replication of DNA.
Besides organelles, the interior of the cell is also compartmentalized in the form of a network consisting of tubules, vesicles and small sacs. This network is known as Endoplasmic Reticulum (E.R.). This endoplasmic reticulum has a continuity with the nuclear membrane.
The space or cavity between the endoplasmic reticulum is known as Cistemae and has a connection with the flattened sacs made of membranes called the Golgi complex (Fig. 1.9). The function of this Golgi complex includes some modification of newly-synthesised macro- molecules and inclusion of these substances into granules for secretion and storage.
There are other membrane-bound vesicles present in the cytoplasm. Lysosomes help in breaking down the foreign materials in the cell by secreting some degradative enzymes. Micro bodies are small vesicles which act in various oxidative metabolisms. Large vesicles, vacuoles are more common in plant cells and act in storing some secondary metabolites and also help in releasing some waste materials.
ii. Functioning as Genetic Material due to the Presence of Nucleus and Ribosomes:
In eukaryotic cells, the genetic material is present in the nucleus (Fig. 1.10) which is enclosed by a membrane. The nuclear membrane is also known as nuclear envelope which consists of two fine layers. The nuclear envelope has a connection with the endoplasmic reticulum although the physical nature of the two are not the same (Fig. 1.11).
The most important difference is the presence of numerous nuclear pores of 50-70 nm in diameter (Fig. 1.12). The nuclear envelope controls the transport of chemical substances from the nucleus to the cytoplasm, and vice versa. It also serves as an anchoring point of chromosomes/chromatin fibres during interphase.
Chromatin fibres or chromosomes, containing the genetic material (DNA), are present in the nucleus. Within the nucleus there is a small spherical structures called nucleoli which varies from 1 to many in several plant materials. The nucleus is filled with a fluid-like substance called the nuclear sap. The nuclear sap contains many solutes and granular particles with a network of fibrous particles called the Nuclear Matrix.
In prokaryotic cells, nucleoli and organised nucleus are absent; the genetic material or DNA are folded into a compact structure known as Nucleoid (Fig. 1.11). There is no membrane separating the nucleoid from the cytoplasm of the cell.
This genetic material, i.e., DNA, both in prokaryotes and eukaryotes, regulates the synthesis of protein on small cytoplasmic structures known as Ribosomes. The ribosomes contain equal amounts of protein and RNA.
The only difference in prokaryotic and eukaryotic ribosomes is in the size, the former is slightly smaller than the latter. The structure and function of the two types of ribosomes are similar. The ribosomes generally occur free in the cytoplasm but some are attached to the endoplasmic reticulum in case of eukaryotic organisms. Some are present in mitochondria and chloroplasts.
iii. Functioning in Cellular Metabolism through Mitochondria and Chloroplasts:
Most of the reactions in metabolism are performed in the cytoplasm’s of the cell. The initial reaction of the cell starts through the degradation of reserve foods in the fluid phase of the cytoplasm known as Cytosol. The main biochemical reaction which requires energy like ATP occur in the cytoplasmic organelles like mitochondria (Fig. 1.14) and chloroplast (Fig. 1.15).
Mitochondria are the site of many chemical reactions, particularly in the position of matrix and Cristae. The matrix of mitochondria also contains DNA and ribosomes. The cristae, the inner folding of the mitochondrial membrane, are the sites of ATP formation.
In case of photosynthetic eukaryotes, the use of energy from sunlight is being done by some specialised organelles called chloroplasts. In case of other photosynthetic organisms like bacteria and blue-green algae, similar trapping of energy from sunlight occur but specialised structures like chloroplasts are not found.
The in-folding of the plasma membrane perform the function of the chloroplasts. Chloroplasts, like mitochondria, are also compartmentalized by forming stroma containing thylakoid membranes having the pigment chlorophyll.
ATP synthesis also occurs in the thylakoid membranes of the chloroplast. The stroma, like the matrix of mitochondria, also contains DNA and ribosomes. It is the site of many biochemical reactions. Thus, both chloroplasts and mitochondria have a genetic autonomy to a certain extent.
iv. Functions Involving Motility Through Microtubules, Microfilaments and Other Bodies:
One of the important characteristic of all cells is movement or motility. It may be either the movement of a cell from one place to another or the movement of liquid over the surface of a cell by cilia and flagella. When the appendages are short and present in large numbers, these are known as cilia. Flagella present in prokaryotes are 10-20 nm in diameter and the internal structure is very simple.
The flagella present in eukaryotic cells are 25-50 times larger in diameter. The internal structure is also very complex having ‘9 + 2’ arrangement of tubules. 2 pairs of inner tubules are surrounded by 9 pairs of outer hollow tubules.
In eukaryotic cells, Microtubules of 25 nm diameter are present which consist of tubulin protein. They play an important role in forming spindle apparatus that helps in the movement of chromosomes into two poles during cell division. Again, microtubules are the main constituent of Cytoskeleton which is a framework or skeleton upon which the different components of the cytoplasm are distributed.
There are other smaller fibres, 6 nm in diameter, termed as Microfilaments, in the eukaryotic cells. The main constituents of these microfilaments are actin protein. The function of microfilaments is in the movement of the plasma membrane during cytokinesis and in producing cytoplasmic streaming. The function of the contractile fibres in muscle cells is done by microfilaments.
Another fibrous structure is present in constructing the cytoskeletal structure of the cells which is known as intermediate filaments. It is so named because the size is between that of microfilaments and microtubules, i.e., 7- 11 nm in diameter.
7. Elemental Composition of Cells:
To understand the function of cells clearly, it is necessary to know the different types of molecules present in the cell. Of the different chemical substances, a relatively small number is most important in performing the essential functions of the living cells.
They are Carbon, Hydrogen, Oxygen, Nitrogen, Phosphorus and Sulphur. These constitute the major components of macromolecules (Table 1.4). Other substances are required for performing other specialised functions.
i. Water:
Water constitutes 75-90% of the total cell mass. Most of the cellular activities are regulated by the water. Most of the chemical reactions in the cell use water as a medium. Water has also a role during hydrolysis and dehydration.
Different types of chemical bonds between atoms and molecules are important in living systems. The strongest and the most stable are the covalent bonds between atoms. Non- covalent bonds—hydrogen bonds, ionic bonds etc.—are weaker but they have a key role in stabilising proteins and nucleic acids.
In a covalent bond the nuclei of two atoms are close together as the electrons in their outermost shells are shared by both. This covalent bond is found in water molecule. In water molecule, electrons are not equally shared between Oxygen and Hydrogen. Two electrons are involved in the formation of a covalent bond, and the other two are associated with the oxygen.
This shows polarity in water molecules having a partial negative charge at one end while the other with partial positive charge. It also forms weak binding of water molecules to each other and with other substances.
So, water also bears another important property of high heat capacity. It means that it requires high amount of heat to change the temperature of water thus giving to the cell an important property of withstanding temperature fluctuations of the environment. The polarity of water also helps to dissociate other molecules into ions.
ii. Other Main Components:
All other main components of the macromolecules play an important role in forming the carbon skeleton. All cells are constructed of organic compounds. These organs are formed from four important compounds—sugars, fatty acids, polysaccharides, amino acids, and nitrogenous bases. These chemical building blocks are responsible for the variegated diversity and complexity of living organisms.
iii. Proteins:
The most structurally and functionally working macromolecules of the cell are proteins. It consists of more than two-thirds of the cell’s total dry weight. They control almost all the chemical reactions of a living organism, regulate the permeability of membranes and control the function of genes.
Each type of protein is formed by joining of amino acids, the building blocks of proteins. There are twenty different amino acids which differ only in their side chains. Each amino acid contains an amino group (-NH2) and a carboxyl group (-COOH). Only Proline has an imino group (-NH-) instead of an amino group. These groups are ionised as -NH3+ , -COO– and NH2+ at a certain pH.
The central carbon atom of all amino acids (carbon) is attached to an amino group, carboxyl group, to a hydrogen atom and to a variable side group or R group:
The classification of amino acids is made according to their electric charge when they are ionised. Lysine, Arginine and Histidine are positively charged. Aspartic and Glutamic acids axe negatively charged.
The joining of amino acids occurs by forming a reaction between the amino group of one amino acid and the carboxyl group of another. This reaction forms a covalent Peptide bond (C-N bond) with the liberation of a water molecule.
A single linear arrangement of amino acids is called a polypeptide. Two amino acids joined in this way form a dipeptide, three a tripeptide and many amino acids a polypeptide. If the polypeptide is short—containing fewer than 30 amino acids long—it is sometimes called oligopeptide.
Each polypeptide has a free amino group at one end (N-terminus) and a free carboxyl group at the other (C-terminus). Some proteins are of few polypeptides but there might be vast number of different kinds of proteins having a combination of 20 amino acids or with different sequence of amino acids.
This different combination of amino acids is responsible for giving the proteins a structural and functional diversity. This complexity in the arrangement of amino acids in a protein makes it difficult to analyse the protein structure. However, the protein structure may be subdivided into primary, secondary, tertiary and quaternary.
a. Primary Structure of Protein:
It refers to the linear arrangement of amino acids in a particular protein. This sequence of amino acids is studied by degrading proteins into peptides (smaller fragments) and then with the help of an instrument—the Amino Acid Analyser.
The structure of Insulin, containing 51 amino acids, was first analysed by F Sanger in 1956. Since that date several proteins have been sequenced to note the relationship between protein structure and function. But proteins do not always remain in a linear arrangement, the interaction between amino acids result in the folding of protein molecules giving a complex structure.
b. Secondary Structure of Protein:
This type of structure is formed by forming hydrogen bond between the -C = O group of one peptide bond and the -NH group of another. Polypeptide chains may run parallel, i.e., in the same direction, or in opposite direction (antiparallel).
Thus, a three-dimensional configuration is formed which is terminated as β-configuration. This secondary structure is exhibited by the fibrous proteins like silk and keratin. .Here two polypeptide chains are held together by hydrogen bonds to form a pleated sheet.
In another type of secondary structure, the polypeptide chains are coiled to a spiral structure due to the formation of hydrogen bonds. This type of structure is known as alpha (a) helix and is found in water-soluble globular proteins. Globular proteins also contain some non-helical regions along with the helical structure. All these structures can be demonstrated through X-ray crystallography of proteins.
c. Tertiary Structure of Protein:
The structure is formed by the folding of polypeptide chains with the formation of weak bonds between amino acid side chains. These bonds are: covalent bonds, ionic interactions, hydrogen bonds and hydrophobic interactions.
The covalent bond is formed between the sulfhydryl groups of cysteine residues. Ionic bonds are formed between positively charged and negatively charged side chains of Lysine, Arginine, Histidine (+) and Glutamic and Aspartic acid (-), respectively.
Hydrogen bond is formed between various side chains of amino acids. Hydrophobic connections are formed between non-polar amino acids like Valine, Leucine, Tryptophan and Phenylalanine. They all give a three-dimensional structure.
d. Quaternary Structure of Protein:
The nature of associations in the formation of bonds is the same as that of tertiary structure. But here the bonds occur between amino acids located in different polypeptide chains. In this way they can hold different chains or sub-units or multiple chains together.
The proteins of high molecular weight above 50,000 show this type of configuration. As for example, Haemoglobin contains four sub-units and bacterial RNA polymerase contains five sub-units. The maintenance of a specific conformation of protein structure leads to the normal function of the proteins.
Any alteration in secondary, tertiary or quaternary structure leads to the loss of functional activity of proteins. This disruption in the structure is known as denaturation. Different substances like Urea, salt and other organic solvents can denature proteins. Increase in temperature also cause denaturation of proteins (Fig. 1.16).
Anfinsen showed in his experiment with Ribenuclease that denatured protein (Ribonuclease) returns to original structure after the removal of denaturing agent, showing the stability of protein configuration. When the protein is treated with denaturing agents like urea, the breakdown of Hydrogen bonds occurs.
Another common denaturing agent is mercaptoethanol which disrupts disulphide bonds causing a loss of normal activity of proteins. The complexity in protein structure allows protein to perform diverse functions in the biological system. The most important function of the protein is the activity of enzymes.
There are other functions too (Table 1.5):
iv. Carbohydrates:
They fall into a group called Polysaccharides containing Carbon, Hydrogen and Oxygen in the ratio of 1 : 2 : 1. The smallest carbohydrates are the simple sugars, i.e., monosaccharide’s of the simple sugars, three carbons (Trioses), five (Pentose’s) and six (Hexoses) are very common in living systems. The most common six-carbon sugar is Glucose which is a key-source of energy in living systems.
Another most important sugar found in all living systems is the Pentose’s, i.e., ribose and deoxyribose—which are the constituent of nucleic acids. The building block of other larger molecules of sugar is the monosaccharide.
When two monosaccharide’s are joined together, disaccharide is formed. Addition of more monosaccharide’s produces Oligosaccharides (oligo = few) and the formation of long chains with many monosaccharide’s is known as Polysaccharides. The common examples of Polysaccharides are cellulose, starch and glycogen. Some complex polysaccharides are formed by joining different kinds of sugar derivatives.
v. Lipids:
These are insoluble in water and are readily extracted by organic solvents like Acetone, Chloroform and Ether. The lipids found in the biological systems can he divided into five types—neutral fats, phospholipids, glycolipids, steroids, and terpenes.
Neutral fats are formed by joining with glycerol and fatty acids. When one fatty acid is joined to one molecule of three-carbon glycerol it is known a monoglycerides. Similarly, di and triglycerides are formed by joining two or three fatty acids with one molecule of glycerol, respectively. These fatty acids may be saturated (all single bonds) or unsaturated (with one or more double bonds).
As there are large number of fatty acids so a large variety of neutral fats are found. The linkage between the fatty acid and glycerol is formed by joining of the carboxyl group of a fatty acid with the hydroxyl group of glycerol liberating a molecule of water during the process. The function of neutral fats is to store energy in the living system.
Phospholipids are a group of lipids where phosphate groups are incorporated into the lipids backbone. Phospholipids have both hydrophilic (water-loving) and hydrophobic (water-hating) properties, these are known as amphipathic molecules.
Out of the three carbons of Glycerol, sometimes phosphate group is attached to one carbon and the rest two carbons are attached with two fatty acids, then this is known as Phosphoglycerides.
Again, different kinds of alcohols may be joined with the phosphate groups. In another type, Sphingophospholipids, the phosphate group is attached to an amino alcohol known as Sphingosine. All these different types of phospholipids play an important role in the structure and function of plasma membranes.
Another important constituent of cell membrane is the Glycolipids, derivatives of Sphingosine, which contains carbohydrate chains attached to lipids by covalent bond. Glycolipids have a variable number of sugars. Fatty acids are present in Glycolipids but there are no phosphate groups. They are the main constituents of membranes.
Steroids axe distinct from other lipids because these are derivatives of phenanthrene ring system. Most steroids function as regulator in cell metabolism—testosterone, estrogen, progesterone, cortisone etc. Another type of steroid, cholesterol, is an important constituent of cell membranes. This also used as a precursor for the synthesis of other steroids.
Another types of lipids derived from the five carbon compound Isoprene were Terpenes. Terpenes are used as building blocks for the production of other substances like Vitamin A, Coenzyme Q and Carotenoids. One of the important property of various types of lipids is the hydrophobic nature.
As they create a barrier to cells between aqueous environments, lipids play an important role in the origin and evolution of cells with a distinct membrane systems.
vi. Nucleic Acids:
Nucleic acids are the molecules that store and transmit information in cells. The genetic information is snooded within the linear sequence of blocks as nucleotides. Each nucleotide is composed of nitrogenous base, pentose sugar and a phosphate group.
Nucleotides may contain one, two or three phosphates and are known as mono-1 di- and triphosphates. When the phosphate group is absent in any nucleotide, it is known as nucleoside.
There are two types of nucleic acids in cells:
Deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Both DNA and RNA are composed of four different nucleotides—DNA contains pentose sugar as Deoxyribose and RNA contains five carbon sugar as Ribose.
The bases present both in DNA and RNA are Adenine (A) and Guanine (G), derivatives of Purine ring and Cytosine (C). Thymine (T) and Uracil (U) are derivatives of pyrimidine. Thymine (T) is present only in DNA while Uracil (U) is present only in RNA instead of T.
The nucleotides—building blocks of nucleic acid—polymerize by joining the phosphate group on the 5′ carbon of one sugar to the hydroxyl group on the 3′ carbon of another sugar, forming a phosphodiester linkage to form nucleic acids. The addition of nucleotides forms a polynucleotide chain with a sugar-phosphate backbone having the bases projecting outward.
The orientation of a nucleic acid chain is one of the important properties of the nucleic acid molecule. DNA can be linear or circular. Viruses have a variety of genomic Structures—linear or circular, single-stranded or double-stranded DNA or RNA. There are certain viruses, such as SV40 DNA tumour virus, which have double-stranded DNA as the carrier of genetic information.
The genome of Escherichia coli, prokaryote, contains circular DNA. There are two types of circular DNA in prokaryotes. When each strand of double- stranded circular DNA molecule is intact, then it is called closed circular DNA. If one of the two strands is broken, it is called an open circle.
Different types of DNA organisation axe shown in Table 1.6:
DNA actually directs the synthesis of the structural proteins and enzymes which are essential for the cell. During transcription, only one of the DNA strand is copied to produce an RNA copy. There are two types of RNA— messenger RNA (mRNA) and structural RNAs (ribosomal RNA and transfer RNA).
Prokaryotic and eukaryotic cells contain large number of messenger RNAs (mRNAs) which are the carrier of genetic information from DNA to produce protein. The ribosomal RNA (rRNAs) and transfer RNA (tRNAs) form the bulk of cellular RNA and mRNA forms only a few per cent of the total cellular RNA.