The following points highlight the top nine features of a bacterial cell. The features are: 1. Pili or Fimbria 2. Flagella and Locomotion 3. Endo-Flagella 4. Glycocalyx 5. Pros-theca 6. Cell Wall 7. Cytoplasmic Membrane 8. The Cytoplasm 9. Spores and Cysts.
Feature # 1. Pili or Fimbriae:
The surface of many bacteria, particularly that of Gram-negative ones, is covered by hollow, more or less straight, extremely thin proteinaceous hair-like appendages, called pili or fimbriae (singular: pilus and fimbria). Their diameter varies from 3 to 25 nm (nanometer: one-thousandth part of μm) and their length may be up to 20μm. The function of this structure is to attach bacterial cells to the substratum or other cells.
Although the terms pili and fimbriae are generally used interchangeably, some microbiologists prefer reserving the term pili only for those appendages which join bacterial cells of opposite mating type for transfer of DNA. Such pili are called sex-pili or F-pili—where F stands for a fertility factor.
The formation of sex pili in the donor cells is controlled by the F-factor. The recipient cells lack sex-pili. Sex pili found in the male (donor) cells of E. coli are longer than fimbriae and their number is only one or two per cell, whereas fimbriae found in these cells are numerous. The F-pili are about 0.5 to 10μm long. Some bacteriophages (bacterial viruses) attach specifically to these pili.
Fimbriae which are numerous are distributed evenly on the cell surface, or they are sometimes restricted to the two poles of cylindrical cells. Many pathogenic bacteria use fimbriae for attachment to the surface of tissues for initiation of infection. The fimbriae of E. coli contain a special protein, called adhesin at the distal end which helps the bacteria to attach to the intestinal wall. E. coli is a normal constituent of the intestinal bacterial flora.
Both pili and fimbriae are made up of a single class of protein known as pilin. Many subunits of pilin are helically arranged around a central hollow core to form a pilus or fimbria. Both pili and fimbriae originate from the cell membrane and run through the cell wall, the outer membrane of Gram-negative bacteria and through the slime covering or capsule, if it is present. Pili or fimbriae play no part in the locomotion of the bacteria. Their main role is attachment and, in case of sex pili, to bring together two cells of opposite sexuality.
Feature # 2. Flagella and Locomotion:
Flagella (singular, flagellum) are the locomotion organs of bacteria. Bacterial flagella are simpler in structure and much thinner than eukaryotic flagella. They help bacteria to swim in liquid medium. Many species of bacteria—both Gram-positive and Gram-negative, including rods, vibrios, spirilli and few cocci—possess flagella.
Flagella generally have a diameter of 0.01 to 0.02 μm and a length of 10 to 20 μm. The thinness of bacterial flagella makes them invisible even under the highest magnification of light microscope. They have to be examined under an electron microscope. However, special staining procedures which make the flagella thicker have been developed to make them visible under light microscope.
Bacterial flagella are composed of thin, long strands of a protein, called flagellin. These proteins give flagella a rigid structure which is helical and cork-screw like. This structure can rotate around its axis, both in a clockwise or in an anticlockwise direction propelling the bacterial cell forward or backward. The bacterial flagella are, therefore, not whip-like structures, as they are in case of eukaryotic cells.
A bacterial flagellum consists of three important parts. The long thread-like helically coiled part of a uniform diameter which extends outward from the cell surface is called the filament. The filament is made from several chains of globular molecules of flagellin which are intertwined to form the hollow helical structure. At the base of the filament is a short sheath-like structure, called a hook. The hook is composed of a different protein, not flagellin.
The hook is slightly wider than the filament. The third part of the flagellum is the basal body which is anchored in the cell wall and cell membrane. The basal body is complex in structure which differs in Gram-negative and Gram-positive bacteria.
In Gram-negative bacteria, the basal body has four rings attached to a central axis, called a rod. In Gram-positive bacteria, there are two rings. The two innermost rings of both types are attached to the cytoplasmic membrane. The other two rings of Gram-negative bacteria are attached to the cell wall and outer membrane.
The structures of basal bodies of gram-negative and gram-positive bacteria are shown in Fig. 2.6:
Bacterial flagella can occasionally break away and can be regenerated. Regeneration occurs at the tip of the broken flagellum by adding newly synthesized flagellin molecules by the cell and transporting them through the hollow core to the tip of the broken flagellum causing it to grow in length.
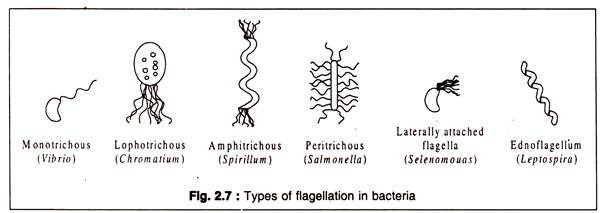
The distribution of flagella on the cell is known as flagellation which is species-specific. There are four major types of flagellation: monotrichous, lophotrichous, amphitrichous and peritrichous. Monotrichous bacteria have a single flagellum attached to one of its pole.
Lophotrichous bacteria have a tuft of flagella at one pole, whereas amphitrichous ones have tufts of flagella at both ends of the cell. Peritrichous types contain flagella spread all over the cell. The four types with an example of each are represented in Fig. 2.7. In some atypical bacteria, like Selenomonas (a vibrio), flagella are present in one side of the curved cell.
The Gram-negative bacterium, Bdellovibrio which parasitizes other bacteria possesses flagella which are enclosed in a sheath which is continuous with the outer membrane. Another very special type of flagella occurs in the spirochaetes. These bacteria have a tuft of flagella beneath the outer membrane and wraps round the cell body in a helical manner. These are known as endo-flagella.
Locomotion of bacteria is due to rotation of the flagella caused by the basal body which acts like a motor. The rings take part in the generation of the rotational force. The motor is driven by the proton motive force or PMF. The force is derived from the electrical potential caused by proton (H+) gradient across the cell membrane.
However, the exact mechanism by which the PMF is converted to the mechanical energy that drives rotation of the basal body resulting in the movement of the flagellar filament is not well understood. It is known that locomotion is an energy-consuming process, though ATP appears not to be directly involved.
Bacterial flagella are rigid, permanently coiled structures which are rotated by the basal body either in a clockwise or anticlockwise manner producing movement, somewhat like the movement of a ship caused by rotation of its propeller.
The velocity of locomotion varies from species to species e.g. Bacillus subtilis which are peritrichously flagellate has an average speed of 27 μm/second, while Vibrio cholerae which are polar flagellate move 200 μm/second.
The speed also depends on the relative viscosity of the medium. In general, a coordinated movement of the bundle of flagella of monotrichous and lophotrichous bacteria in one direction pushes the bacteria forward.
When the direction of the movement is reversed, bacteria move in an opposite direction. This is called a ‘run’. In the change-over period, between the runs, the bacteria ‘tumble’. In peritrichous bacteria, all the flagella rotate synchronously to form a bundle resulting in ‘run’.
Locomotion and the flagellar rotation are shown in Fig. 2.8:
Why do bacteria have locomotion? One of the obvious answers is that locomotion enables them to keep themselves under optimal physical and chemical conditions. Bacteria often show a directed movement either towards or away from a physical or chemical stimulus. In other words, the response to a stimulus may be positive or negative. This type of movement is known in biology as taxis.
Depending on the nature of the stimulus, several types of tactic movements are known, such as chemo-taxis, photo-taxi.s, aero-taxis etc.. Depending on the nature of the organism, the same stimulus may produce opposite responses. This was demonstrated by the pioneer microbiologist Beijerinck in a simple but elegant experiment.
A drop of a suspension of three different types of motile bacteria was placed on three slides and covered by a coverslip. The bacteria were allowed to move to their optimal oxygen concentration. It was found that strongly aerobic bacteria collected in large numbers at the margin of the coverslip. The anaerobic bacteria moved to the centre where the oxygen concentration was lowest, while the bacteria which are not highly aerobic (microaerophilic) collected at some distance from the margin (Fig. 2.9).
From the above simple experiment, it is obvious that bacteria are capable of regulating their flagellar movement in response to a stimulus (in this case oxygen)—either positively or negatively. This raises the question: how do they sense or perceive a stimulus? This has been partly understood in case of chemo-taxis.
It has been observed that bacteria exhibit a directed movement (a run) towards a chemical attractant only when the attractant is present in a concentration gradient and the bacteria swim from a lower concentration towards a higher one without much ‘tumbles’. If the bacteria are placed in a homogeneous medium (i.e. without concentration gradient), they exhibit a non-directed movement of short runs and tumbling’s.
For exhibiting chemo-taxis, bacteria have a sensory system consisting of chemoreceptors which are special type of proteins located in the periplasm (the space between outer membrane and cell wall of Gram-negative bacteria) and another type of proteins, called transducers which are located in the cytoplasmic membrane. The chemoreceptors receive the chemical stimulus and transmit a signal to the transducers.
The transducers transmit the signal to the flagellar motor through some small cytoplasmic proteins. The transducer proteins can undergo methylation and de-methylation and this process seems to control the direction of rotation of the flagellar motor. Methylation of transducers activates the cytoplasmic proteins by phosphorylation. At least two cytoplasmic proteins appear to be involved.
The sensory apparatus is schematically represented in Fig. 2.10:
Fig. 2.10: Sensory system for chemical stimulus
A very special type of tactic movement shown by some bacteria is their response to a magnetic field. This response is called magneto-taxis. A bacterium exhibiting magneto-taxis is Aquaspirillum magnetotacticum. The cells contain a chain of magnetite (Fe3O4 ) particles in a body called a magneto-some. The magneto-some acts like a magnet and confers the ability of these bacteria to orient themselves as magnetic objects in the magnetic field of the earth.
A magnified image of A. magnetotacticum is drawn in Fig. 2.11:
Feature # 3. Endo-Flagella:
Spirochaetes show a characteristic type of motility with the help of internally borne flagella-like structures, called endo-flagella or axial filaments. A bundle of these structures are anchored at one end of the thin long cell body.
The filaments wrap around the cell in a helical manner and remain throughout the length covered by the outer membrane of the cell envelope i.e. the flagellar filaments are restricted within the periplasmic space. Therefore, endo-flagella are also known as periplasmic flagella (Fig. 2.12).
The endo-flagella do not differ significantly from the normal prokaryotic flagella in their ultrastructure. They contain a basal body having the rings of typical Gram-negative type. But the characteristic wreathing movement of spirochaetes is quite different from the movement of other bacteria.
It appears that the spirochaetal movement also is caused by rotation of the axial filaments generated by the basal body. The rotation causes the outer membrane of spirochaetes to rotate in a cork-screw like manner moving the cell in a liquid medium. However, a peculiar feature is that spirochaetes show better motility in comparatively viscous liquid media.
Gliding Movements:
Some non-flagellate bacteria, such as the cytophagas and myxo-bacteria, exhibit a slow gliding movement only on solid surfaces. The mechanism of such movement is not known. Slow excretion of slimes through minute pores has been proposed by some to be the force driving the bacteria forward in a zigzag manner.
Feature # 4. Glycocalyx:
Many bacteria possess an extracellular highly hydrophilic layer composed mostly of polysaccharides and occasionally of polypeptides, or of both. The general term used for this covering is glycocalyx. A gelatinous cover layer sticking firmly to the cell and having a well-organized structure is called a capsule. A loose covering which diffuses into the surrounding medium is called a slime layer. The chemical components of glycocalyx are synthesized by the cell and are transported across the cell membrane and the cell wall and deposited outside to form the extracellular covering.
The glycocalyx polysaccharides contain many different sugars and sugar acids, such as glucose, galactose, rhamnose, fucose, mannose, glucuronic acid, etc. Streptococcus pneumoniae has a capsule composed of glucose and glucuronic acid, alternating with each other. Leuconostoc mesenteroides, a lactic acid bacterium, produces enormous amount of slime consisting of dextran when the bacteria grow in a medium containing sucrose.
Dextran is a polysaccharide of α-D-glucose having 1,6-glycosidic bonds. Streptococcus (Lactococcus) salivarius produces a polymer of fructose, called laevan. Zoogloea ramigera produces slime layers which coalesce to form a homogeneous mass within which the individual bacteria are held together to form a natural colony.
Many species of Bacillus, such as B. megatarium, B. subtilis, B. anthracis etc. possess well defined capsules which are composed of polypeptides. The polypeptides consists of D- and L- amino acids. Some bacteria like Acetobacter xylinum and Sarcina ventriculi produce extracellular cellulose, which is a very uncommon polysaccharide for bacteria. The typical packets of cells of Sarcina ventriculi are held together by cellulose. However, cellulose is not considered to form a Capsule in strict sense.
Several other bacteria produce extracellular heteropolysaccharides to form a well-defined sheath enclosing a series of individual cells resulting in a filamentous structure. Examples of such sheathed bacteria are Spherotilus natans present in sewage tanks and Leptothrix ochracea. Gallionella and Nevskia also produce profuse polysaccharides in the form of stalks which hold together the cells.
The presence of a well-defined capsule can be demonstrated by mounting a drop of bacterial suspension in Indian ink. As the carbon particles of the ink do not penetrate the capsule, the bacteria are seen to be embedded in a dark background.
This is called negative staining (Fig. 2.13):
The capsule or slime layer is not essential for the life of the bacteria, because mutants lacking a capsule can grow normally. But these structures play an important role when the bacteria grow under natural conditions. One of the major functions of capsule appears to be protection against engulfment by phagocytosis. Predators of bacteria, like protozoa and amoebae feed on bacteria by phagocytosis.
Similarity, the phagocytes of animal bodies, like leucocytes and macrophages kill pathogenic bacteria by phagocytosis. A well-known example is that of pneumococci. Capsulated pneumococci can escape phagocytosis and are pathogenic, while the non-capsulated variants are non-pathogenic, because they are easily devoured by the phagocytic cells of the human body.
The presence of a capsule also protects the bacterial cells from desiccation under natural conditions. But a more important role of the glycocalyx is attachment of bacterial cells to solid substratum under conditions where they may be washed away by a flowing stream.
Streptococcus mutans, an organism associated with dental caries attaches to the dental surface with the help of a glycocalyx consisting of a water-insoluble polymer, glucan. Bacteria sometimes form multilayered colonies, called biofilms, with the help of glycocalyx.
The first layer of cells attach to a solid surface with the help of fimbriae or pili. The succeeding layers adhere to the first layer with the surface polysaccharides to cement the layers together to form a biofilm. Biofilms in pipelines produce acids with damaging effect. Microbial biofilms are of considerable economic importance.
Feature # 5. Pros-Theca:
A pros-theca is defined as a semi-rigid appendage extending from a bacterial cell. The appendage has a diameter less than that of the cell and it is covered by a cell wall. A pros-theca is an extension of the main cell and its cytoplasmic content is continuous with that of the cell.
A pros-theca is sometimes called a stalk; but the term stalk should be preferably restricted to appendages formed by excreted polysaccharides as found in Gallionella, Nevskia etc. Stalks are made up of non-living materials and not integral parts of the cell, as prosthecae are.
Prosthecae are used mainly for two purposes, — attachment or reproduction by budding. In genera like Caulobacter, Ancalomicrobium, Asticcacaulis etc. the pros-theca serves to attach the bacteria to some solid substratum or to other cells. Caulobacter has a single pros-theca with a knob-like structure at the distal end which helps in attachment. Ancalomicrobium cells have several prosthecae (Fig. 2.14).
Some other genera—Hyphomicrobium, Rhodomicrobium, Pedomicrvbium etc.—use the prosthecae to produce a bud at the distal end. The bud develops into a cell which may break away to produces a new bacterium, or sometimes the bud develops into a new cell which also produces a pros-theca and a bud, resulting into a colony of cells connected with each other by the prosthecae. In Rhodomicrobium vannielii, the daughter cells produced by budding do not separate and may form a branched colony.
Feature # 6. Cell Wall:
With very few exceptions, all bacterial cells are provided with a rigid wall. Hans Christian Gram (1853-1938) in 1884 developed a staining procedure by means of which bacteria could be differentiated into two groups.
The procedure named after him is known as Gram staining. It consisted of treating a bacterial smear with a basic dye, like crystal violet, followed by a dilute iodine solution as a mordant. The bacteria absorbed the stain and were coloured deep violet.
Next, the bacterial smear was treated with ethyl alcohol or acetone. This treatment produced two different types of responses depending on the nature of the bacterium smeared. In one response, the bacteria were decolorized by alcohol or acetone. These bacteria were designated as Gram-negative. The other response was opposite, that is, the bacteria retained crystal violet stain after washing with alcohol.
They were designated as Gram-positive. This differential behaviour was later found to be due to basic differences in the structure of the cell wall. Not only that, the response to Gram staining has been found to be associated with many other properties. Hence, Gram staining is of fundamental importance in the study of bacteria.
The cell wall is more or less a rigid covering of the inner protoplasm. The wall gives protection to the protoplast and gives the cell a definite shape. Because bacteria generally grow in hypotonic (this means that the osmotic pressure of the cell is higher than that of surrounding medium) environment, the protoplast would burst had there been no wall to limit its expansion.
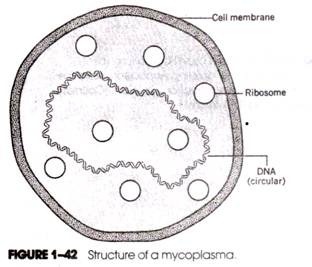
This can be demonstrated by suspending protoplasts produced by enzymatic removal of the cell wall in a hypotonic medium. At the same time, protoplasts generated from cylindrical cells are spherical. This means that the characteristic shape of bacteria is due to the wall. This is also supported by the lack of definite shape in mycoplasmas which are cell wall-less bacteria. Similarly, the L-forms (L stands for Lister Institute where they were first observed) of bacteria induced by treatment with inhibitors of cell wall synthesis are also devoid of any characteristic shape.
The cell wall of bacteria does not act as a barrier of entry of dissolved salts, sugars and most of the low molecular weight compounds. The permeability barrier of the cell is the cell membrane. Depending on the species, cell wall may account for 10 to 40% of the dry weight of bacteria.
On the average, about 20% of dry weight of bacterial cells is cell wall. A basic constituent of the bacterial wall, except that of the taxonomically distinct archaebacteria, is a unique polymer called peptidoglycan or murein. Murein forms a macromolecular sac-like covering of the inner contents of the bacterial cell. Murein is mainly responsible for the rigidity of the wall.
The backbone of murein is made of long chains of two alternating molecules, an amino sugar, called N-acetyl glucosamine (NAG) and its lactyl ether, called N-acetyl muramic acid (NAM). NAG and NAM are joined with 1,4-glycosidic bond.
In murein, the lactyl group of NAM residues are mostly linked with short chains of amino acids (tetra-peptides). Parallel peptidoglycan chains are usually linked through other peptides. The repeating units of E. coli murein with tetra-peptide chain is shown in Fig. 2.15.
The cross-linking of two parallel chains of peptidoglycan is shown in Fig. 2.16:
Cell wall of Gram-positive and Gram-negative bacteria differs in chemical structure. The murein content of Gram-positive cell wall is higher, ranging between 30 to 70% of the dry weight of the cell wall. In Gram-negative wall, murein is on the average about 10%. The murein sac of Gram-positive bacteria is many layered (up to 40). Each layer consists of parallel chains of peptidoglycan which are cross-linked.
Several such layers are laid one above the other to form a thick sheet in which the peptidoglycan chains are cross linked. In Gram-negative bacteria, the number of peptidoglycan layers is one to few. Besides, the cell envelope of Gram-negative bacteria is more complex than the wall of Gram-positive bacteria.
A simplified representation of the differences between gram-positive and gram-negative wall structure is shown in Fig. 2.17:
(i) Gram-Positive Cell Wall:
The multilayered peptidoglycan coat forms a thick, rigid covering of the Gram-positive bacterial cell. Generally, this coat is externally covered by a tightly packed protein layer, called the S-layer (surface layer). In addition, many Gram-positive bacteria contain other polymers in their cell wall. One of these polymers is teichoic acid consisting of ribitol phosphate or glycerol phosphate (Fig. 2.18). They are found in Staphylococcus aureus, Bacillus spp., Streptococcus faecalis etc.
Another polymer, teichuronic acid, is present in the cell wall of some Gram-positive bacteria, like Bacillus licheniformis. Teichuronic acid consists of long chains of alternating glucuronic acid and N-acetylgalactosamine linked to each other by 1—>3 glycosidic bond (Fig. 2.19).
Some teichoic acid molecules are anchored in the cytoplasmic membrane. These are called lipoteichoic acids. Other teichoic acids are bound to the N-acetyl muramic acid residues of the peptidoglycan chains by phosphodiester bonds. Both types form long chains which project outside the cell wall. Because of the presence of phosphate groups, teichoic acid molecules are negatively charged.
They probably regulate movement of cations (positively charged ions) and play an important role in holding Mg++ in the walls of Gram-positive bacteria. Depending on the pH and salt concentration, the teichoic acid molecules can change in conformation. Besides teichoic and teichuronic acids, other polymers, like covalently linked polysaccharides, may be present in association with the peptidoglycan layers e.g. in Streptococcus pyogenes.
In Gram-positive bacteria, the peptidoglycan chains contain the tetra-peptides consisting of L-lysine-D alanine-L-lysine and D-alanine residues. In some members, e.g. Staphylococcus aureus, the tetra-peptide chains are not directly interlinked as in Gram-negative bacteria, but are connected through pentaglycine chains through an L-lysine residue of one tetra-peptide chain and the D-alanine residue of another chain (Fig. 2.20).
Some Gram-positive bacteria, particularly Mycobacterium tuberculosis and M. leprae—the causative organisms for tuberculosis and leprosy, respectively—show the property of acid-fastness. When these bacteria are stained with carbol-fuchsin and then washed with dilute acid, the bacteria retain the stain. Non-acid fast organisms are de-stained by a similar treatment. The property of acid- fastness has been found to be due to the presence of a lipid, called mycolic acid in the cell walls.
(ii) Gram-Negative Cell Wall:
The most distinctive feature of the Gram-negative cell wall is the presence of an outer membrane. The space between the cytoplasmic membrane and the outer membrane is known as the periplasmic space. The other features are that the peptidoglycan layer is much thinner consisting of one to few layers, and that the teichoic or teichuronic acid chains are absent.
The peptidoglycan chains are not as compact as they are in Gram-positive bacteria. They are connected to the outer membrane. The periplasmic space, which includes the peptidoglycan chains, has an aqueous gel-like consistency and it contains a variety of proteins having either transport functions or degradation enzymes, like β-lactamase which destroy antibiotics of β-lactam group, such as penicillins, cephalosporin’s etc.
Like other membranes, the outer membrane is a bilayered structure. The main components of the outer membrane are lipopolysaccharides, lipoproteins and phospholipids. The presence of these lipids makes the Gram-negative cell walls rich in lipids which account for 11 to 22% of the dry weight of the wall.
The lipopolysaccharides (LPS) form the outer layer of the bilayered outer membrane. The LPS has three components, — lipid A embedded in the outer membrane, core polysaccharide lying on the membrane surface, and polysaccharide side-chains (O-antigen) projecting outside the membrane, like whiskers. These O-antigens are specific for different strains and are useful for taxonomic identification.
The LPS of Gram-negative bacteria acts as their endotoxins. The LPS layer of the outer membrane is interrupted here and there by the presence of fine pores which are formed by a special protein, called porin. These pores allow entry of many nutrient molecules into the periplasmic space and fr0m there into the cytoplasm. Different porins allow only specific kinds of molecules to pass through. Large molecules, like those of proteins, cannot pass through these pores.
The outer membrane also contains several other types of proteins. Some protein molecules span through the entire outer membrane. These are known as receptor proteins. Others are smaller lipoproteins, called Braun’s lipoproteins, restricted to the inner layer. These are mainly involved in anchoring the peptidoglycan layers to the outer membrane.
The peptidoglycan of Gram-negative bacteria is more or less similar to that of Gram-positive bacteria, except that the tetra-peptide chain contains meso-diaminopimelic acid (m-DAP) in place of L-lysine and that the pentapeptide (pentaglycine) chains are absent. The tetra-peptide tails attached to NAM are directly cross linked between m-DAP and D-alanine of adjacent peptidoglycan chains as shown in Fig. 2.16.
The outer membrane thus serves as a permeability barrier of Gram-negative bacteria. It allows entry of smaller molecules, like those of sugars, amino acids or even nucleosides through the porin-channels and receptor proteins. At the same time, it prevents escape of enzymes and other macromolecules from the periplasmic space. On lysis of Gram-negative bacteria, the LPS acts as powerful endotoxins causing pathological symptoms in the host organism.
The presence of an outer membrane also protects the bacteria from the action of lysozyme, an enzyme produced by many microorganisms as well as human beings. This enzyme present, in saliva, tears and other body fluids can specifically attack the glycosidic bond between NAG and NAM and thereby causes destruction of peptidoglycan chains. While lysozyme can attack readily Gram-positive bacteria, the Gram- negative bacteria become susceptible only when the outer membrane is sufficiently damaged allowing lysozyme to enter and—react with the peptidoglycan layer.
The site of action of lysozyme on peptidoglycan is shown in Fig. 2.22:
A diagrammatic representation of a cross-sectional view of the Gram-negative cell wall is shown in Fig. 2.23:
(iii) Differential Response to Gram Stain:
Difference in the cell wall structure of Gram-positive and Gram-negative bacteria is responsible for their response to Gram staining. The crystal violet-iodine complex is deposited on the cytoplasmic membrane. Washing with alcohol removes the stain in Gram-negative bacteria, because the thin peptidoglycan layer cannot prevent the stain being washed out.
The much thicker layer of peptidoglycans of Gram-positive cell wall does not allow the escape of the stain complex. It has been found that when Gram stained Gram-positive bacteria are treated with lysozyme, the wall is removed but the crystal violet-iodine complex still remains adsorbed to the cell membrane. When these are next washed with alcohol, the dye-complex is easily washed out. This proves that the presence of the murein holds back the dye- complex from being washed out by alcohol.
(iv) Cell Wall of Archaebacteria:
Archaebacteria constitute a taxonomically distinct group of bacteria which differ in several important characteristics from the rest of prokaryotic organisms. One of the distinctive features of archaebacteria is the absence of murein in their cell wall.
Archaebacteria can be Gram-positive or Gram-negative. Some members do not have a cell wall and are comparable to the mycoplasmas of true bacteria (eubacteria). The Gram-positive archaebacteria have a comparatively thick cell wall composed of complex polymers, mostly polysaccharides. Some of the Gram-positive methanogenic (forms methane) archaebacteria, like Meihanobacterium, have a cell wall composed of a peptidoglycan-like polymer, called pseudomurein.
It consists of repeating units of N- acetyl glucosamine and N-acetyl talosamino uronic acid joined to each other by β-1,3 glycosidic bonds (Fig. 2.24). The chains of this polymer are cross-linked by peptides containing only L-amino acids. The D-amino acids characteristically present in eubacterial murein are absent in archaebacterial cell wall. The Gram-negative archaebacteria have cell walls of variable composition. Mostly the wall is composed of one or two layers of proteins or glycoproteins.
Feature # 7. Cytoplasmic Membrane:
The limiting membrane of the protoplast is the cell membrane or plasma membrane present in all biological cells including bacteria. On its outside is the cell wall—if present—and towards the inside is the cytoplasm including its different components and inclusions.
The cell membrane is about 7.5 nm thick and is composed of phospholipids accounting for 20 to 30% and proteins accounting for 60 to 70% by dry weight. The phospholipid molecules form two layers in which the hydrophilic (polar) ends of the lipids are on either side and the hydrophobic (non-polar) long chain fatty acid ends project inwards to form a hydrophobic zone.
The membrane proteins are of two types. Some are intimately bound to the lipids and others are loosely bound to the periphery of the lipid bilayer. They are designated as membrane-integral proteins and peripheral proteins, respectively.
The membrane-integral proteins generally span through the lipid bilayers and they are bound to the fatty acid chains through hydrophobic amino acid residues. The peripheral proteins are generally bound to the hydrophilic layer of membrane by charge interaction. They can be comparatively easily separated from the membrane lipids, like washing with salt solutions.
A schematic representation of the cytoplasmic membrane is shown in Fig. 2.25:
The cytoplasmic membrane has been envisaged as a semi-fluid structure consisting of a mosaic of lipids and proteins (fluid mosaic model). The globular protein molecules are thought to float about in the lipid matrix of the membrane.
The fluidity of the membrane has been thought to be essential for its functions. The presence of saturated and unsaturated fatty acids in the phospholipids is thought to be a determining factor for the degree of fluidity of the membrane.
The prokaryotic cell membranes do not contain sterols, except that of some mycoplasmas. In the absence of cell all, sterols are supposed to give some rigidity to the cell membrane of mycoplasmas enabling them to resist lysis against higher osmotic pressure.
As shown in Fig. 2.25, the interior of the membrane bilayer is a strongly hydrophobic zone which prevents entry of water molecules and water soluble compounds. The presence of specific proteins in the membrane makes the entry of such polar molecule possible into the cytoplasm. Thus, while the membrane acts as an effective barrier for entry and escape of nutrient molecules, the membrane proteins serve as portals of regulated transport in both directions.
The cell membrane is semi-permeable and acts as an osmotic barrier. The membrane proteins function as transporting agents, each protein can transport one or a few selected molecules. A very important function of the bacterial cell membrane is that in absence of mitochondria in prokaryotic cell, it performs as the site of energy generation through electron transport system (ETS).
The ETS localized in the cell membrane expels protons outside the membrane, creating a proton motive force (PMF) which is utilized to drive ATP generation from ADP and inorganic phosphate. Another function of the bacterial membrane is that it houses certain proteins and factors which are involved in the translocation of the precursors of peptidoglycan. An important factor is a carrier called bactoprenol, a long chain (C 55) alcohol. It carries peptidoglycan units through membrane to the site of peptidoglycan synthesis.
The cell membrane of Gram-negative bacteria also functions in transporting outer membrane proteins. Similarly, the extra-cellular proteins, including exotoxins, are liberated from the cells of many Gram-positive bacteria. These exportable proteins are synthesized within the cell and trans-located outside through the membrane. So, it is observed that the bacterial cell membrane, besides being an osmotic barrier, performs many other useful functions.
(i) Archaebacterial Cell Membrane:
The structure of cell membrane of archaebacteria differs in several important features from the membrane of eubacteria. In eubacteria, the membrane lipids are composed of straight chain saturated and unsaturated fatty acids bound to glycerol phosphate by ester bonds i.e. the carboxyl -COOH group of fatty acids is linked with the hydroxyl -OH groups of glycerol by an ester bond. The membranes of archaebacteria contain branched chain alcohols bound to glycerol phosphate by ether linkage. The long chain alcohol is often phytanol, a C 20 alcohol.
Sometimes, two phytanol molecules are joined end to end to form a more stable structure (Fig. 2.26):
Archaebacterial membrane also contains other lipids which are unsaturated compounds. A predominant lipid of this type is squalene, a C 30 isoprenoid compound.
Feature # 8. The Cytoplasm:
The main cell body, covered externally by the cell membrane, is generally known as the protoplast. It contains both particulate bodies and various soluble ingredients essential for the life of the cell. When bacterial cells are disintegrated artificially and centrifuged at high speed (~ 100,000 x g) for several hours, a deposit containing the insoluble particulate ingredients and a supernatant containing the soluble ingredients are obtained.
The soluble fraction represents the cytosol and it contains various enzymes, soluble RNAs and a host of organic and inorganic substances. The particulate fraction contains the remnants of cell wall, cell membrane, ribosomes, nuclear material and various non-living inclusions.
An ultra-thin section of bacterial cell (fixed with osmium tetroxide and stained with uranyl acetate), made by ultra-microtome and observed under transmission electron microscope reveals a central electron-transparent zone where the nuclear material is located. This central zone is not separated from the rest of the cytoplasm by any membrane. The cytoplasm appears as electron-dense and granular. This portion of the cell contains most of the ribosome and various inclusions.
The bacterial cell does not have a cytoskeleton consisting of microtubules and membrane bound organelles, like mitochondria, plastids, lysosomes etc. characteristically present in eukaryotic cells. However, some bacteria, specially the photosynthetic ones, contain intracytoplasmic membrane systems which are produced by invagination or in-folding of the cytoplasmic membrane.
In photosynthetic bacteria, these extended portions of the cell membrane contain the photosynthetic pigments, as well as the photosynthetic energy producing systems (phoienhosphorylation). These intracytoplasmic membrane systems are called chromatophores and they may be of various shape and size. In some non-photosynthetic bacteria also, e.g. Nitrosococcus (a chemoautotroph, and Azotobacter (a diazotroph), presence of elaborate intracytoplasmic membrane system has been observed.
Another controversial structure, called meso-some—supposed to be produced by invagination of the cytoplasmic membrane—is found in many bacteria, specially the Gram-positive ones. This structure has been implicated to play a role in replication of DNA and in export of extracellular enzymes.
The main constituents of the cytoplasm are discussed in more details below:
(i) Ribosomes:
Ribosomes are ribonucleoprotein-containing particulate bodies which serve as the sites of protein synthesis. An actively growing bacterial cell contains thousands of ribosomes which impart the granular appearance of the cytoplasm. During protein synthesis, ribosomes form a chain connected by the m-RNA which is being translated. Such strings of ribosomes are known as polysomes.
A prokaryotic ribosome is somewhat smaller than an eukaryotic ribosome. It measures about 16 x 18 nm and contains about 60% RNA and 40% protein. About 80-85% of the total RNA of bacterial cell is present in the ribosome. This RNA is known as ribosomal RNA (r-RNA).
The intact ribosomes can be precipitated by ultra-centrifugation at a sedimentation rate of 70 Svedberg units and, therefore, they are known as 70S ribosomes. The eukaryotic ribosomes which are larger are 80S ribosomes.
The sedimentation rate of particulate bodies is determined by the size, density and shape of the bodies. Besides bacteria, 70S ribosomes are also present in the cell-organelles of eukaryotic cells, like mitochondria and chloroplasts. This indicates that these organelles probably originated from prokaryotic cells (endosymbiotic hypothesis).
Like eukaryotic ribosomes, the prokaryotic counterparts also consist of two subunits. In prokaryotic ribosomes, the two subunits have sedimentation constants of 50S and 30S. In eukaryotes, they are 60S and 40S. When 70S ribosomes are subjected to low Mg++ concentration, they dissociate into 50S and 30S subunits which are commonly known as large and small subunits, respectively.
The 30S subunit consists of 21 different proteins and a single kind of RNA, — 16S RNA. The 50S subunit contains 34 proteins and two kinds of RNA’s, — 5S and 23S.
The gross structures of 30S, 50S and 70S ribosomes are shown in Fig. 2.27:
The 40S eukaryotic ribosomal subunit has 33 different proteins and one 18S RNA. The 60S subunit has 49 proteins and three kinds of RNA, —5S, 5.8S and 28S. These differences between prokaryotic and eukaryotic ribosomes have been profitably exploited for selective inhibition of bacterial protein synthesis by antibacterial agents.
For example, streptomycin inhibits bacterial protein synthesis by binding to 30S subunit and chloramphenicol to the 50S subunit. Eukaryotic ribosomal subunits do not bind to these agents and hence these antibiotics do not affect protein synthesis of hosts, like human beings.
(ii) Bacterial Nucleus (Nucleoid):
The more or less electron transparent area occupying the central portion of the cytoplasm is the nuclear area. It is filled with a fibrillar material and can be stained by the Feulgen reagent which is specific for DNA. The nuclear material of prokaryotes consists of a single, long, supercoiled, circular, double-stranded DNA molecule which is associated With RNA and some proteins.
This large super molecule is called the bacterial chromosome or nucleoid. The E. coli chromosome is 1.2 mm long when fully extended, i.e. more than 1,500 times longer than the cell itself. It contains 4.7×106 base-pairs. This long molecule is super-coiled to be packaged into an intracellular space occupying only about 20% of the cell volume of an average E. coli cell.
The super-coiling is induced by an enzyme called topoisomerase. However, super-coiling is not enough for packaging DNA in a small volume of the nuclear area. The DNA associated proteins play an important role to hold the large molecule in 40 to 50 independently super-coiled loops to form the nucleoid (Fig. 2.28).
(iii) Cytoplasmic Inclusions:
Depending on the conditions of growth, bacteria may possess different non-living inclusions, most of them being insoluble metabolic products. Volutin granules are found in many bacteria e.g. Spirillum volutans. Volutin granules are insoluble polyphosphates and can act as source of reserve phosphate. Another very common reserve substance is a polymer of β-hydroxybutyric acid (PHB), a fat-like sub- stance. It acts as a reserve source of carbon and energy.
In some bacteria, PHB granules may fill the major part of the cytoplasm and may account for up to 80% of the dry weight of bacteria. Reserve carbohydrates in the form of insoluble glycogen are found in many bacteria, like E. coli, Bacillus polymyxa. Micrococcus luteus etc. Also, a starch-like compound occurs in some bacteria. It is called granulose.
Sulfur granules are found in many photosynthetic bacteria, like Chromatium spp., as well as in some non- photosynthetic ones, like Beggiatoa spp., Thiothrix sp. etc. Some species of Bacillus like B. thuringiensis contain a crystalline protein, called a parasporal body, which possesses insecticidal property.
Structures of some of the reserve substances and parasporal protein are shown in Fig. 2.29.:
Some aquatic bacteria, specially the green and brown photosynthetic bacteria, like Amoebobacter, Pelodictyon etc. possess gas vacuoles or airosomes. These bodies are made up of parallelly arranged hollow gas-filled cylinders with conical ends and bound by a protein membrane. Gas vacuoles help the cells to maintain buoyancy and to float at an optimum depth in the aquatic environment.
(iv) Carboxysomes:
Carboxysomes are cytoplasmic particulate bodies found in some autotrophic bacteria like Nitrosomonas, Nitrosococcus, Thiobacillus etc. and also in the cyanobacteria including Prochloron. These bodies contain the CO2-fixing enzyme ribulose bisphosphate carboxylase (rubisco).
(v) Magnetosomes:
In connection with tactic locomotion of bacteria that certain bacteria can show directed movement in response to a magnetic field, e.g. Aquaspirillum magnetotacticum by virtue of the presence of magnetite particles (Fe3O4). These particles are in the cytoplasmic bodies called magnetosomes. Magnetosomes are able to split hydrogen peroxide.
They help the bacteria to move downward, so that the bacteria can reach an optimal environment. In such anaerobic environment hydrogen peroxide—which is toxic—may be produced. Magnetosomes may have a protective role by splitting the toxic peroxide.
Feature # 9. Spores and Cysts:
Although spore formation is not prevalent in the common bacteria, specialized groups are characterized by the ability to form spores of several types. Cyst formation is still rarer in bacteria. The spore-forming genera may form spores within a cell, usually singly.
These spores are called endospores. They are formed typically by all members of the genera Bacillus and Clostridium. A few other bacteria belonging to Sporolactobacillus, Desulfotomaculum, Thermoactinomyces and Sporosarcina are also known to form endospores.
Spores are also formed outside the cells by some bacteria, particularly the mycelium-forming actinomycetes. These bacteria, like many fungi, produce spores externally either singly or in chains. Such spores are known as conidia. In some genera of actinomycetes, conidia are formed within sporangia. Such spores are generally known as sporangiospores.
A group of bacteria, known as myxobacteria, are characterized by formation of micro-fruit bodies and myxospores. Myxobacteria may also form myxospores without fruit bodies. Myxospores are also known as micro-cysts. Cysts are rare in bacteria. A cyst is a dormant resting cell produced by differentiation of an entire bacterium. Cysts are typically found in some species of the nitrogen-fixing genus Azotobacter.
(i) Endospores:
The most characteristic type of bacterial spores is the endospores. The property of endospore formation is restricted to some aerobic and anaerobic bacterial genera. The great practical importance of these genera is due to the ability of the endospores to withstand extreme environmental stresses, particularly temperature.
Endospores are also highly resistant to UV-irradiation, desiccation and high vacuum. An extreme example of their survival has been the report that endospores trapped in amber believed to be 25 million year old retain their viability.
Several dangerous pathogenic bacteria belong to the endospore-forming genera, like Bacillus anthracis cause of anthrax, Clostridium tetani— cause of tetanus, C. perfringens—cause of gangrene, and C. botulinum—the agent causing botulism poisoning.
It has been suggested that the retention of viability of endospores for over millions of years makes them as possible “seeds” which might have travelled through interstellar molecular clouds from one planet to another. But more down-to-earth importance of the endospore-forming pathogens is that they are very difficult to kill.
Endospores can survive boiling for several hours. So, for elimination of such dangerous bacteria, like C. tetani, and C. botulinum, the temperature has to be raised to a higher level and kept at that temperature for sufficiently long time.
As each bacterial cell forms a single endospore, it is clear that endospores do not serve as a means for multiplication. Their extreme resistance to stresses suggests that endospores are resting forms meant for tiding over the adverse environmental conditions. Endospores germinate on return of congenial conditions, each endospore producing a single vegetative cell.
Under light microscope, a suspension of spore-forming bacteria reveals the endospores as highly retractile objects, because of their low moisture content and high content of proteinaceous materials. The endospores can be differentially stained with malachite green and carbol fuchsin.
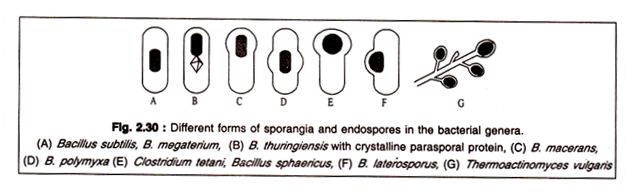
The mother cell within which the spore has been formed is generally called a sporangium. A sporangium may have different forms depending on the species. It may retain the shape of the vegetative cell or may be swollen in the portion where the spore is formed. The spore may be formed centrally, terminally or sub-terminally. The spores themselves may have different shape and size.
Different forms of sporangia are shown in Fig. 2.30.:
Transmission electron microscopy of ultra-thin section of endospores reveals complex structural features. The main spore body is externally covered by a loose structure called exosporium which is formed by the remnants of the mother cell protoplast.
Inside the exosporium are several layers of spore coats. Next to the coat layers is a thick zone, called cortex. Below the cortex lies the primordial cell wall enclosing the inner membrane. The inner membrane surrounds the core or protoplast of the spore.
The structural features of an endospore are shown in Fig. 2.31:
Endospore formation:
Endospore formation is initiated in a vegetative cell when the normal growth is limited by an essential metabolite, particularly the carbon and energy source. The nuclear material assumes an axially disposed filamentous structure and the vegetative cell are partitioned asymmetrically by a double membrane septum formed near one pole.
The nuclear material divides into two complements, one remaining in the mother cell and the other in the smaller compartment. Only the double membrane separates the two compartments. The peptidoglycan layer of the original cell does not take part.
At the next stage, the smaller compartment is engulfed within the larger compartment by gradual proliferation of the septal membrane and advancement of the membrane towards the pole. After the engulfment is completed, the smaller compartment—which now becomes a fore-spore—lies embedded within the cytoplasm of the mother cell. The fore-spore is covered by two membranes, the inner fore-spore membrane (ifm) and the outer fore-spore membrane (ofm).
Next, the cortex is built up by deposition of spore-specific peptidoglycan between ifm and ofm. The cortex becomes thick and on, maturity forms the major part of the spore. The cortex is a distinctive structure of bacterial endospore. Its peptidoglycan differs from that of the wall peptidoglycan in being less cross-linked and, therefore, a more lax or loose structure than the more compact wall peptidoglycan.
Moreover, the accessory polymers like teichoic acids are absent in cortical peptidoglycans. Also, whereas most N-acetyl muramic acid residues contain tetra peptide tails in the wall peptidoglycans, in cortex only about one-third of these residues have a tetra peptide tail. The inner layers of the cortical peptidoglycan gives rise to the primordial cell wall which in mature spore forms the cell wall.
The two membranes, ifm and ofm, of the fore-spore are unique in having opposite polarity i.e. the outer surfaces of the two membrane face each other, while the inner surfaces are on the two external sides. Such orientation of the ifm and ofm results automatically from the engulfment process of the fore-spore by the mother cell (Fig. 2.32). The opposite orientation has important bearing on the transport of metabolites into the fore-spore.
The development of endospores is depicted in Fig. 2.33 below:
An important feature of endospore formation is the accumulation of Ca++ in the mother cell. In vegetative cells, Ca++ level is low, but, during sporulation, Ca++ is actively transported from the medium to reach a concentration of 3 to 9 mM.
From the mother cell cytoplasm, Ca++ is transported by facilitated diffusion into the fore spore. The fore spore contains a unique compound, dipicolinic acid which is absent in the mother cell. This compound (Fig. 2.34) has the property of chelating Ca++ to form calcium dipicolinate. The concentration of calcium dipicolinate may be so high that it constitutes 10-15% of the dry weight of the endospore. This compound has been implicated with the heat resistance of the bacterial endospores.
If endospore formation takes place in media containing low calcium concentration, spores are less thermo-resistant. But a more important factor conferring heat resistance is the highly dehydrated state of the cytoplasm of the mature spores. This factor is also responsible for high refractive index of the spores making them highly retractile granules under light microscope.
Endospores are liberated by disintegration of the sporangia. Some parts of the mother cell cytoplasm remain adhered to the spore-coat forming the loose exosporium. Germination of endospores begins by production of a germ tube either through one pole, or sometimes laterally. The germ tube divides to produce a vegetative cell. During germination, water is rapidly imbibed with simultaneous loss of heat resistance. Germinating spores rapidly lose dry weight to the extent of 25 to 30%. The loss of dry weight is due to excretion of amino acids, peptides and dipicolinic acid.
Germination of endospore is shown in Fig. 2.35:
(ii) Bacterial Exospores:
Formation of single exospores by unicellular bacteria is found only in very few instances. A methane-oxidising bacterium, Methylosinus, has been reported to form single spores outside the cell by budding. These exospores are also heat and desiccation resistant, but they do not contain dipicolinic acid.
Photosynthetic bacterium, Rhodomicrobium vannielii, is also known to form triangular exospores under depleted nutritional conditions. These exospores are also heat, desiccation and UV-resistant. The triangular exospores germinate by producing germ tubes from each corner on return of favourable nutritional conditions (Fig. 2.36).
Conidia are formed externally and found in most species of actinomycetes. Actinomycetes, as already noted, are mycelium forming bacteria, sometimes with elaborate substrate and aerial hyphae. In the members of Streptomyces, Micromonospora, Streptosporangium, Actinoplanes etc. conidia are formed in the aerial hyphae. In many cases, there are long straight, flexuous, spiral or coiled chains of conidia formed from the tip of conidiophores. In some genera, conidia are formed enclosed in a sporangium (Fig. 2.37).
The characteristic smell emanating from soil following a shower has been found to be due to the dispersed conidia of soil actinomycetes. Conidia are generally desiccation resistant, but not thermo- resistant.
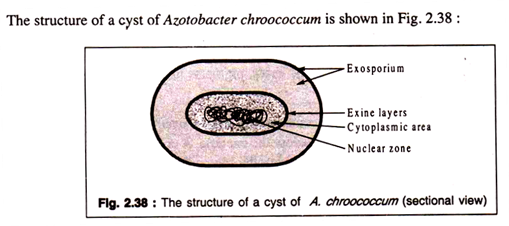
(iii) Cysts:
Cysts are thick-walled, desiccation-resistant resting stages, common among protozoa and amoebae, but also found in a few bacteria. Some species of Azotobacter—nitrogen-fixing bacteria— present in soil and aquatic environment are characterized by formation of well-defined resting structures, called cysts.
A bacterial cyst is a thick-walled structure provided with an exosporium and an exine containing within a cytoplasmic portion with nuclear material, ribosomes etc. A vegetative cell is converted into a cyst by investing the protoplast with two layers of wall and a thick exosporium.