In this article we will discuss about the physical and chemical properties of amino acids.
Important Physical Properties of Amino Acids:
1. Optical Isomerism:
All amino acids, except glycine which has two H atoms on the α-carbon, have an asymmetric α-carbon (the 4 valence bonds are attached to different atoms or groups). As may be seen in figure 1-1, one can therefore write — for example, in the case of alanine — the spatial formula of two optical isomers or enantiomers (which form a racemate when mixed), non-superimposable because one is a plane mirror image of the other, and whose physical and chemical properties are identical (except the rotatory power).
One therefore distinguishes for each amino acid, an isomer D, and an isomer L, depending on whether the spatial structure is identical to that of D glyceraldehyde or L glyceraldehyde (whose absolute configuration is known).
The spatial formulae being rather complicated, the plane projection formulae proposed by Fischer is preferred; by convention one writes the amino acids of the D-series with the amino group on the right, and those of the L-series with the NH2 on the left.
It may be noted that the prefixes D and L have absolutely no significance relative to the direction in which the plane of polarized light is rotated by the amino acid. This direction may be specified by a sign (+) or (-) placed before the name of the amino acid [Example: L (―) leucine], keeping however in mind the fact that the rotatory power changes with the conditions of the medium, particularly the pH.
All amino acids constituting the proteins belong to the L-series. But in various bacterial peptides, particularly those present in the cell wall, or certain peptides having an antibiotic activity, one finds amino acids of the D-series.
On the other hand, certain amino acids have an additional asymmetric carbon in the side chain, which increases the possibilities of isomerism; thus for threonine, one will have D and L threonine and D and L allothreonine.
Let us note that in organic chemistry, the designations L and D are now being replaced by the letters S (latin “Sinistrum”: left) and R (“Rectum”: right). Example: S-alanine (L) and R-alanine (D).
2. Absorption in the Ultraviolet Region:
Amino acids present an appreciable absorption at wavelengths below 230 nm (2 300 Å); furthermore, some of them absorb between 250 and 300 nm, due to the presence of chromophores like the phenyl radical (Tyr) or the indole ring (Trp) in their R chain, thus permitting the spectrophotometric titration of proteins.
3. Ionization:
All amino acids contain at least two ionizable groups, the carboxyl group and the amino group; they are amphoteric; the carboxyl group of an amino acid can yield one proton, and an anion appears:
The amino group can fix one proton and form a cation.
These two dissociation reactions correspond to equilibria governed by the law of mass action; the proportions of ionized and unionized amino acid present in solution will therefore depend on the concentration of H+ ions.
The two dissociation constants K1 and K2, corresponding to the two equilibria, may therefore be written as follows:
The exact values of K1 and K2 vary for different substances but it may be said that K1 for a carboxylic acid is of the order of 10-4 to 10-6 (1) and K2 for an amine, between 10-8 and 10-10. Knowing the values of K1 and K2 we can calculate for each H+ ion concentration (i.e. for each pH), the percentage of ionized molecules.
For example, at pH 7, one will have for the carboxyl group (taking for K1 a mean value of 1 x 10-s):
This means that at pH 7, there will be only one non-ionized molecule for 100 anion molecules, in other words, the percentage of ionization of the acid group is 99%.
For the amino group, at the same pH 7, one will have (taking for K2 a mean value of 1 x 10-9):
At this pH, the percentage of ionization of the amino group is therefore 99%.
When the H+ ion concentration is equal to K1, we have [R-COOH]/[R — COO–] = 1, in other words, the number of anions present is equal to the number of non- ionized molecules.
If, as in the example given above, K1 = 10-5, we also have [H+] = 10-5, and this situation is therefore reached at pH = 5, i.e. when the pH is equal to pK (-log K) of the ionizable group. The pK corresponds to the pH of half dissociation, it indicates the ease with which this dissociation takes place and it is a measure of the strength of the acid.
The same is true when the H+ ion concentration is equal to K2. One then has [R-NH2]/[R—NH3+] = 1, i.e. cations and non-ionized molecules in equal number, and when K1 = 10-9 (above example) this situation is reached at pH = 9. Here again, the pH of half dissociation is called pK and is a measure of the strength of the base.
When the pH of a solution of an amino acid is raised from a low value to a high value, one has the following transformations:
It may be noted that at a particular pH, the amino acid molecules are in the dipolar form (Zwitterion) and the net charge of the molecule is nil; this is the isoionic or isoelectric point of the amino acid. At this pH, its solubility is minimal and it does not migrate when placed in an electric field (unlike the cation and the anion).
The dissociation of the various polar groups of an amino acid may be easily studied by adding HCl or NaOH to the solution and measuring the pH after each addition. We can thus plot titration curves, of varying shapes depending on whether the amino acid is neutral, acidic or basic.
A. Titration of a Neutral Amino Acid:
When acid (HCl) is added, the base R —COO– is titrated (according to Bronsted’s definition, a base is a compound capable of capturing one proton):
When a base (NaOH) is added, the weak acid R – NH3+ is titrated (an acid, because it is capable of yielding one proton):
It is observed (see fig. 1-2a) that there are 2 zones where the addition of HCl or NaOH produces only a very small variation of the pH; there is therefore a buffer effect; glycine is actually used to form a glycine-HCl buffer (where glycine is in the form of hydrochloride) and a glycine-NaOH buffer (where glycine is in the form of sodium salt).
At the middle of each of these 2 buffer zones, one has the pH value corresponding to the pK of the 2 groups: pK1 (half dissociation of the carboxyl group) = 2.3 approximately, and pK2 (half dissociation of the amine) = 9.7 approximately. For comparison, the pK of acetic acid is 4.8: it therefore appears that the carboxyl group of glycine is an acid 100 times stronger than that of acetic acid, which is due to the effect of the α-amino group.
The isoelectric pH or pHi = pK1 + pK2/2 = 2.3 + 9.7/2 = 6. Figure 1-2a shows that this pHi is at the centre of an isoelectric zone. For a pH = pK1 + 2 (here 2.3 + 2 = 4.3), the acidic group is ionized to 99% and for a pH = pK2 – 2 (here 9.7 – 2 = 7.7), the amino group is ionized to 99%; one may therefore consider that in the pH zone between 4.3 and 7.7, glycine is almost entirely in zwitterion state.
B. Titration of a Dicarboxylic Amino Acid:
The titration curve of aspartic acid is shown in figure 1-2b. If the solution is strongly acidic the aspartic acid will be entirely in the protonated form; one adds sodium hydroxide which will react with all the-protons supplied by the amino acid (the 2 carboxyl groups and the protonated amino group — NH3+ are all acids according to Bronsted’s definition, because they can all yield a proton). During the titration by sodium hydroxide one will therefore have the different forms represented in figure 1-3.
The pK values corresponding to the half dissociations of the 3 protons are respectively about 2.1,3.9 and 9.8. It is seen that the most acidic carboxyl group is the one carried by the α-carbon because it is near the — NH3+ which, by repelling positive charges, promotes the dissociation of the α-carboxylic group.
At pH 2.1 one has, in equal quantities, the form having a net charge + 1 and the one having a net charge 0. Similarly, at pH 3.9, one will have in equal quantities the form of charge 0 and that of charge -1. The form of net charge 0 will be predominant at pH 2.9 which is the isoelectric pH of aspartic acid.
C. Titration of a Basic Amino Acid:
We have not shown the titration curve of a basic amino acid but one can have an idea of it if one knows the pK values of lysine and arginine:
From what we have just seen, it appears that if one has a mixture of amino acids in a solution at pH 6 for example:
1. Neutral amino acids like glycine will be in the form of zwitterion +A– (charge = 0),
2. Acidic amino acids like aspartic acid will be in the form +A2- (charge = -1),
3. Basic amino acids like lysine will be in the form 2+A– (charge = +1).
This explains why amino acids can be separated by fractionation methods based on charge differences, especially electrophoresis and ion exchange chromatography.
So far, we have only referred to carboxylic and amino groups but one finds in amino acids other groups which also can be ionized. Besides the guanidium group of arginine we just mentioned, we may cite the imidazole nitrogen of histidine (pK = 6.7).
In proteins, except at each end, the α-NH2 and α-COOH are all involved in the peptide bonds and therefore cannot be ionized. Hence, the ionizable groups of the side chains mainly contribute towards conferring a charge to the macromolecule (i.e. the groups we just mentioned and those of the basic and dicarboxylic amino acids).
Depending on the pH, a given protein may have a positive charge, a zero charge (when the + and – charges balance each other), or a negative charge. For a given protein, one will therefore have an isoelectric point at which the protein will not migrate if it is placed in an electric field; this pH, will be low if the protein has an excess of carboxylic groups; it will be high in the case of a protein having an excess of amino groups.
Important Chemical Properties of Amino Acids:
The chemical properties of amino acids are due to the carboxyl group, the amino group and the radical R. We shall mention only the important properties, particularly those referred to later while studying the peptides and proteins.
1. Reactions Due to the Presence of the Carboxyl Group:
A. Formation of Salts:
This property may be used for titrating the amino acids, but due to the weak dissociation of the carboxyl group, high pH values (around 11 or 12) must be reached to obtain total saturation (except when titration is carried out in presence of formaldehyde).
B. Decarboxylation:
It can be carried out in the laboratory, but it takes place by enzyme action in living cells.
2. Reaction Due to the Presence of the Amino Group:
A. N-Alkylation and N-Aryiation:
The two H of NH2 can be replaced by:
i. Aliphatic radicals, the simplest of which is CH3; N-methyl, N-dimethyl and N-trimethyl derivatives can be obtained (refer to glycine, for example; figure 7-10).
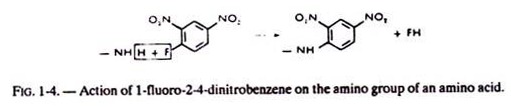
ii. Aromatic radicals like the dinitrophenyl (DNP) derivatives used for the determination of the N-terminal amino acid of proteins. For example, one can see in figure 1-4, the action of 1 fluoro -2-4-dinitro-benzene on an amino-group.
B. N-Acylation:
Formyl, acetyl (easily hydrolyzable) or carbobenzoxyl radicals (C6H5 — CH2—O —CO —, easily eliminated by hydrogenation) are used in the laboratory to ensure a temporary blockage of the amino group.
In vivo, one knows the N-acetyl and N-benzoyl derivatives of glycine (see figure 7-12) formed during detoxication processes. But special mention must be made of N-formyl-methionine which plays an important part in the initiation of protein biosynthesis in bacteria and organelles.
C. Action of Formaldehyde:
Formaldehyde (or methanal) reacts with the NH2 of amino acids at room temperature and neutral pH to form dihydroxymethyl derivatives (figure 1-5) which are now tertiary amines and no longer primary amines; these are weaker bases, which have a lower pK, and one can neutralize the amino acids with a base in presence of phenolphtalein (i.e. without the necessity of reaching pH values as high as in the absence of formaldehyde). This is called Sorensen’s formaldehyde titration.
D. Action of Nitrous Acid:
Figure 1-6 shows that nitrous acid reacts with the NH2 groups, liberating nitrogen which can be titrated. This is the principle of the Van Slyke method for the determination of the free NH2 groups of amino acids, peptides or proteins. This method enables us to follow step by step the hydrolysis of a protein (because as hydrolysis progresses, the number of free NH2 groups increases).
E. Deamination:
Deamination and transamination, causing the removal of the amino group, are two very important enzyme reactions.
3. Reactions Necessitating the Simultaneous Presence of a Carboxyl Group and an Amine in α Position:
A. Reaction with Ninhydrin:
As can be seen in figure 1-7, this reaction consists of several steps: at first we observe the formation of reduced ninhydrin while the amino acid is transformed into an aminoacid; the latter is hydrolysed into an α-keto-acid which is decarboxylated into an aldehyde having one atom less than the starting amino acid; the amino acid can be determined by identification of the aldehyde obtained.
On the other hand, Van Slyke developed a titration of free a-amino acids based on the measurement of CO2 liberated. Then, NH3 produced in the first part of the reaction reacts with one molecule of reduced ninhydrin (also formed during the first step) and one molecule of oxidized ninhydrin, to yield a product of violet-blue colour.
This coloration may either be used to identify the amino acids after paper or plate chromatography or electrophoresis, or, it may be used in certain conditions for a colorimetric titration of amino acids, for example, at the exit of an ion exchange chromatographic column having fractionated a mixture of amino acids.
B. Substituted Amide Linkage between 2 Amino Acids:
A substituted amide linkage or peptide bond (-CO-NH-) can take place between 2 amino acids, the α-COOH of one of them being linked to the α-NH2 of the other.
It will be seen that this linkage joining the amino acids is found in peptides and proteins. If the linkage involves a COOH or a NH2 in another position (β-COOH, γ-COOH, ε-NH2) it is said to be a peplidoid linkage (for example, the linkage between glutamic acid and cysteine in glutathione, see. fig. 1-12).
One can obtain in the laboratory diketopiperazines (see fig 1-8) or cyclic dipeptides, where the α-NH2 of amino acid 1 is involved in a peptide linkage with the α-COOH of amino acid 2 and the α-COOH of amino acid 1 is similarly linked with the α-NH2 of amino acid 2.
4. Properties of the R Radicals:
Since most α-COOH and α-NH2 groups are involved in peptide linkages, it is evident that the chemical properties of proteins are mainly due to the R radicals. The reactivity of these radicals is often attenuated by steric hindrance or by their participation in the three-dimensional configuration of proteins, but it is useful to know the important properties of these side chains.
The alcoholic hydroxyls can be esterified, for example, by phosphoric acid (phospho-serine) or they can be acylated (the OH of tyrosine also).
The aromatic ring permits substitution reactions.
Example:
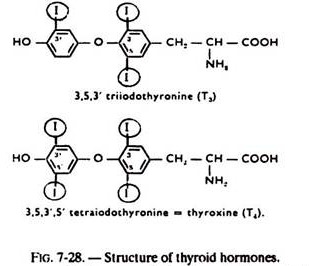
The iodinated derivatives of tyrosine which form the thyroid hormones (see fig. 7-28).
The thiol groups of cysteine can be oxidized and the cysteine-cystine system (2R —SH ↔ R —S —S —R) can act as hydrogen donor or acceptor. The disulphide bridges are important for the three-dimensional structure of proteins. Besides, oxidation can proceed up to the stage R — SO3H (sulphonic acid, whose decarboxylation gives taurine).
Furthermore, the hydrogen of the SH group is a mobile H which can be substituted by acyl radicals, thus forming thio-esters of formula R – S — CO — R’, with high reactivity and of very great importance in biochemistry (example: the S-acylated derivatives of co-enzyme A).