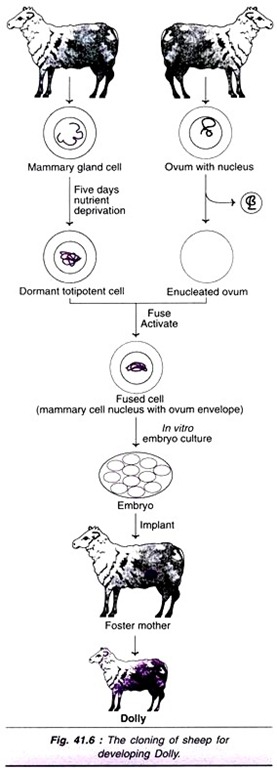
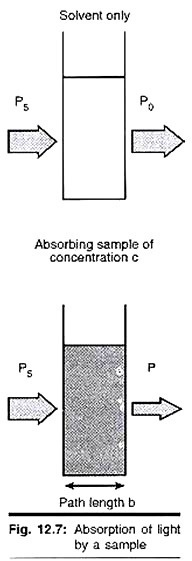
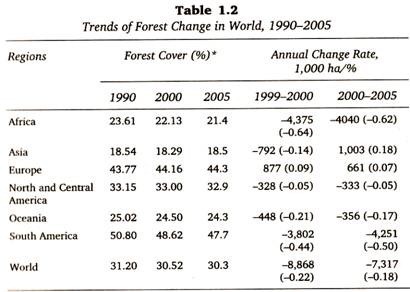
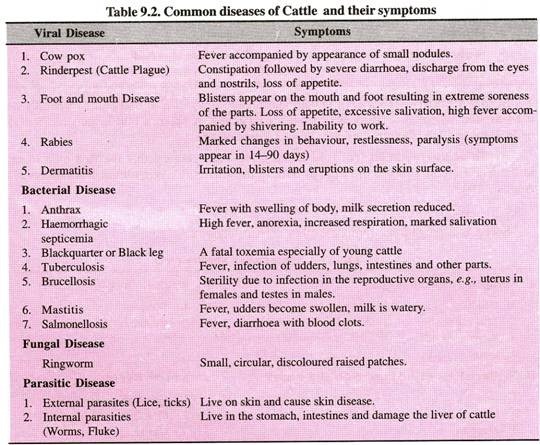
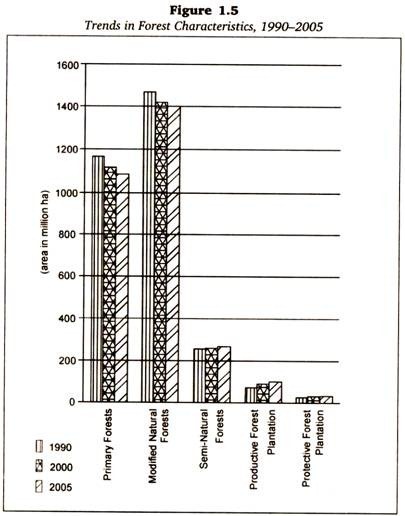
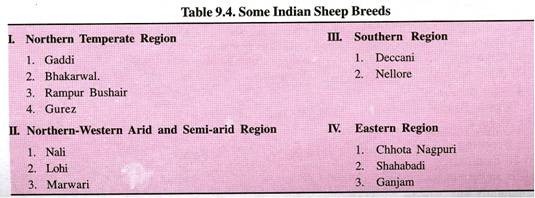
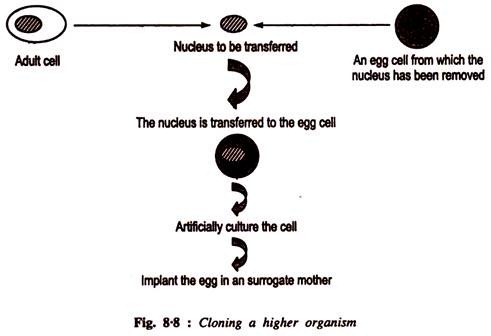
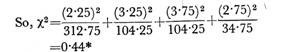Let us make an in-depth study of the amino acids. After reading this article you will learn about: 1. Concept of Amino Acids 2. Classification of Amino Acids and 3. Reactions of Amino Acids.
Concept of Amino Acids:
Amino acids are the building blocks of proteins. Among the thousands of amino acids available in nature, proteins contain only 20 different kinds of amino acids, all of them are L-alpha-amino acids. The same 20 standard amino acids make proteins in all the living cells, may it either be a virus, bacteria, yeast, plant or human cell. These 20 amino acids combine in different sequences and numbers to form various kinds of proteins.
The number of proteins that can be had from these 20 amino acids can be calculated from 20 factorial, i.e., 20 x 19 x 18 x 17 x 16 x ………… x 2 x 1 = 2.4 x 1018. In human beings alone there are more than 100 000 different types of proteins.
The general formulae for an amino acid can be written as ‘R-CH-NH2—COOH’. Depending upon the ‘R’ group present in the amino acid it is named accordingly. The 20 amino acids found in the proteins are known as primary or standard amino acids. In addition to these, some other amino acids are also found in proteins like 4-hydroxyproline, 5-hydroxylysine, 6-IV-methyllysine, gamma carboxyglutamic acid and desmosine, all of these are derivatives of standard amino acids.
Classification of Amino Acids:
I. Depending upon the Charge:
Amino acids can be broadly classified into three major groups:
(1) Neutral
(2) Acidic and
(3) Basic.
1. Neutral amino acids:
Those amino acids that do not contain any charge on the ‘R’ group.
They are further classified into the following categories:
(a) Aliphatic:
Those amino acids whose ‘R’ group contains a chain of carbon atoms—Gly, Ala, Ser, Thr, Val, Leu, lie, Asn, Gin.
(b) Aromatic:
Those amino acids whose ‘R’ group has a benzene ring—Phe, Tyr, Trp.
(c) Heterocyclic:
The “R” group has a heterocylic ring, i.e., any of the ring structures which contain different atoms—Pro, His.
(d) Sulphur containing:
Those amino acids which contain a sulphur atom-Cys, Met.
2. Acidic amino acids:
Those amino acids that contain a negative charge or an acidic group-Asp, Glu.
3. Basic amino acids:
Those amino acids that contain a positive charge or a basic group-Arg, Lys and His.
II. Depending upon the Solubility in Water:
The amino acids can also be grouped into two different categories, depending upon their solubility in water. They are—
1. Hydrophobic amino acids:
Amino acids insoluble in water are known as hydrophobic amino acids. They are—Ala, Val, Leu, lie, Pro, Met, Phe, Trp.
2. Hydrophilic amino acids:
Amino acids soluble in water are known as hydrophilic amino acids. They are—Gly, Ser, Thr, Cys, Tyr, Asp, Asn, Glu, Gin, Lys, Arg, His.
III. Depending upon their Nutritional Requirements:
The amino acids are classified into two groups.
They are:
1. Essential amino acids:
Are those which cannot be synthesized by the human body and hence they should be taken through the diet. There are 10 essential amino acids. Among these amino acids, arginine and histidine are known as semi-essential amino acids.
2. Non-essential amino acids:
These acids are those that can be synthesized in the human body and are not required in the diet. These include gly, ala, ser, pro, tyr, cys, asp, asn, glu, gin.
Reactions of Amino Acids:
Physical Characters of Amino Acids:
1. Zwitter ions:
Amino acids have an acidic group (—COOH group), i.e., a proton, donor. They also have a basic group (—NH2 group), i.e., a proton, acceptor. A compound capable of both donating and accepting protons and thus able to act either as an acid or a base is known as amphoteric molecule. Amino acids have both anions and cations in solution and such compounds are called zwitter ions.
2. Isoelectric pH (pH1):
The pH at which the positive charge on the amino acid (or any other molecule) is equal to the negative charges, is known as isoelectric pH. At this pH the net charge will be zero and hence it does not move either to positive (anode) or to negative (cathode) electrode, when subjected to an electric field. At pH1 all the molecules exists in zwitter ion form.
Chemical Properties:
1. Reactions due to amino group:
(a) Ninhydrin test:
This test identifies or detects amino acids. If amino acids are heated with ninhydrin, they form a purple blue coloured compound, which is measured colorimetrically.
(b) Reaction with nitrous acid:
It is a method by which amino acids are measured depending upon the amount of nitrogen released.
(c) Reaction with carbonyl compounds (RCHO):
The amino group in the amino acids reacts with carbonyl compounds forming a Schiff’s base.
(d) Reaction with Sanger’s reagent:
Amino acids react with Sanger’s reagent, i.e., 1-fluoro-2, 4-dinitrobenzene, forming a yellow coloured complex. This reagent is used to detect the IV-terminal amino acid in the proteins.
(e) Edmann’s reaction:
Edmann’s reagent is phenyl isothiocyanate, which is also used to detect the N-terminal amino acid in a protein. It forms a purple coloured derivative.
(f) Reaction with dansyl chloride:
Dansyl chloride, i.e., 1-dimethyl-amino-naphthalene-5-sulphonyl chloride forms a fluorescent derivate of the N-terminal amino acid of proteins. This is yet another reagent available for the detection of N-terminal amino acid.
(g) Condensation of two amino acids to form diketopiperazine:
Two amino acids react with amino groups of each amino acid and the carboxylic groups of the other amino acid forming a diketopiperazine.
2. Reaction due to carboxylic:
(a) Reaction with hydrazine:
Hydrazine is used to detect the C-terminal amino acid in proteins. It forms a complex with the amino acid by reacting with the carboxylic group.
3. Reaction due to both amino and carboxylic group:
Due to the presence of both amino (basic) and carboxylic (acid) groups in amino acids, the amino group of one amino acid reacts with the carboxylic group of another amino acid to form a peptide bond.
Polymerization of amino acids in a similar manner gives a polypeptide chain.
Peptide bond:
The bond linking two amino acids is known as a peptide bond. It is formed due to reaction between an amino group of one amino acid and carboxylic group of another amino acid.
Peptide group:
The group forming the peptide bond is known as peptide group. It has a double bond character and hence is very rigid in nature.
Polypeptide or peptide:
A chain made up of two or more amino acids, linked by a peptide bond is known as a polypeptide or just a peptide.
Difference between a peptide and a protein:
A peptide is that which has less than 50 amino acids or whose molecular weight is less than 5000 Daltons. A protein is that which has more than 50 amino acids or whose molecular weight is more than 5000 Daltons. This differentiation is based upon the immunological property of the two units. Peptides are non-immunogenic, whereas proteins are immunogenic.
N-terminal and C-terminal of a protein:
The end of a protein or polypeptide where the amino group is free in known as N-terminal end and that amino acid whose amino group is free is known as N-terminal amino acid. Sanger’s, Edmann’s and Dansyl chloride are the reagents used to determine the N-terminal amino acids.
The end of the protein or polypeptide whose carboxylic group is free is known as C-terminal end and that amino acid whose carboxylic group is free in the protein is known as C-terminal amino acid. Hydrazine is used to detect the C-terminal amino acid. While representing a protein on paper, the N-terminal amino acid is written first (on the left) and the C-terminal amino acid is the last one (written at the right side of the paper).
Peptides of physiological importance:
(a) Glutathione:
It is a tripeptide made up of Glu, Cys and Gly. It is found in RBC and other tissues and functions to prevent oxidation of —SH groups of many enzymes.
(b) Bradykinin and kallidin:
These are small polypeptides containing 9 and 10 amino acids respectively. They are formed by partial hydrolysis of plasma protein due to snake poisoning (venom). They are powerful vasodepressors and inhibitors of heart function. Others are tyrocidin, gramicidin, glucagon, insulin, oxytocin, etc.
Structure of protein:
The structure of protein can be studied under four different levels of organization, viz., primary, secondary, tertiary and quaternary.