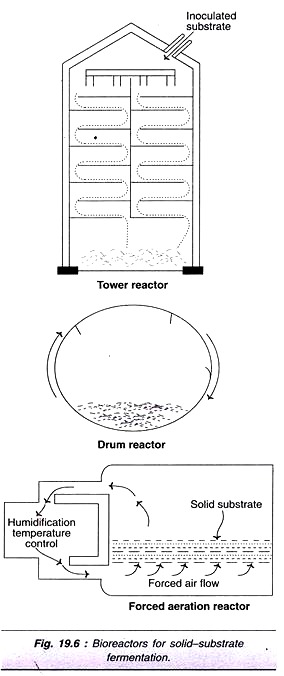
The below mentioned article provides a study note on the Amino Acids.
They are organic acids (with carboxylic group —COOH) having amino group (—NH2) generally attached to a-carbon or carbon next to the carboxylic group.
Carboxylic group provides an acidic property to the amino acid while amino group gives it a basic reaction. The a-carbon also bears a variable hydrocarbon or alkyl group R and hydrogen. Amino acids are, therefore, substituted methane’s where the four substituent groups occupy the four valency positions.
R is represented by H in glycine, methyl group in alanine, hydroxymethyl in serine. In others it may be straight or branched hydrocarbon chain or a cyclic group. The hydrocarbon may further be polar (e.g., serine, glutamate or glutamic acid) or nonpolar (e.g., alanine).
In proline and hydroxyproline, amino group (—NH2) is replaced by imino group (>NH) which also represents the tail of R-group. Proline and hydroxyproline are called heterocyclic amino acids.
Except glycine, a-carbon is asymmetric and the amino acids are generally laevorotatory. Certain non-protein amino acids are dextrorotatory. Twenty types of amino acids and amides occur in proteins (Table 9.3). They are called protein amino acids. Incorporation of protein amino acids is controlled by triplet codes of DNA/mRNA.
A protein may also possess non-coded amino acids. The latter are called rare amino acids. Rare amino acids are derived from the coded ones through modifications, e.g., hydroxyproline from proline, hydroxylysine from lysine (both in collagen). Amino acid names are abbreviated with the help of first three letters of the name. Changes are made for amino acid derivatives like Asn for Asparagine (an amide).
Non-protein amino acids are many in number. They occur both in the free state as well as in combined state, but not in proteins. Some of these are found in antibiotics and are called amino acid analogues. Other non-protein amino acids take part in important biosynthetic pathways.
For example, ornithine and citrulline are involved in urea cycle. Homo-arginine and diaminobutyric acid are a source of nitrogen. Gamma-amino butyric acid (GABA) is an inhibitory neurotransmitter in brain and some other parts. Diaminopimelic acid is an intermediate of lysine synthesis.
All amino acids contain С, H, О and N. Some additionally contain sulphur. Physical and chemical properties of amino acids are mainly due to the amino, carboxyl and R functional groups.
Based on comparative number of amino and carboxyl groups, amino acids can be acidic, basic or neutral. Ring structure in R-groups makes certain amino acids aromatic. Depending upon structure and reaction, amino acids are differentiated into seven types.
(a) Neutral Amino Acids:
Amino acids have one amino group and one carboxylic group (mono-amino monocarboxylic) with noncyclic hydrocarbon chain, e.g., glycine, alanine, valine.
(b) Acidic Amino Acids:
The amino acids have an extra carboxylic group (mono-amino dicarboxylic), e.g., glutamate (glutamic acid), asparate (aspartic acid).
(c) Basic Amino Acids:
They have an additional amino group without forming amides (diamino monocarboxylic), e.g., arginine, lysine.
(d) Sulphur Containing Amino Acids:
The amino acids possess sulphur, e.g., cysteine, methionine.
(e) Alcoholic Amino Acids:
They are amino acids having alcoholic or hydroxyl group, e.g., serine, threonine.
(f) Aromatic Amino Acids:
They possess cyclic structure with a straight side chain bearing carboxylic and amino group, e.g., phenylalanine, tryptophan (actually heterocyclic) or tyrosine (having OH group).
(g) Heterocyclic Amino Acids:
They have nitrogen in the ring structure, e.g., histidine, proline.
A particular property of amino acids is the ionizable nature of NH2 and COOH groups. Hence in solutions of different pH, the structure of amino acids changes.
Plants can synthesise all the protein amino acids required by them. Animals cannot manufacture their own amino acids from raw materials. For this they are dependent upon the plants directly or indirectly. Animals, however, have the ability to form several amino acids through transformation or transamination. They cannot synthesise seven of the protein amino acids.
The amino acids which cannot be synthesised by animals through transformation or transamination are called essential amino acids. These amino acids must be present in their diet, viz-, leucine, isoleucine, valine, tryptophan, phenylalanine, lysine and methionine.
Human adults require an additional essential amino acid named threonine while children need two more (arginine and histidine). The latter are named as semi-essential amino acids.
Peptide Formation:
Amino acids condense to produce peptides. Generally condensation reaction occurs between a primer amino acid or peptide and another amino acid. Molecule of water is eliminated. The bond thus formed —NHCO— is actually amide bond which is popularly known as peptide bond or linkage.
Chain containing two amino acids linked by a peptide bond is called dipeptide. There are three amino acids in a tripep- tide, a few in oligopeptide and numerous in a polypeptide.
The term peptide is more commonly used instead of oligopeptide. Like an amino acid a peptide has carboxylic group at one end and amino group at the other end. Occasionally the peptide is acidic or basic due to the presence of specific amino acids.