Vaucheria: Occurrence, Reproduction and Life Cycle!
Contents
Vaucheria:
Systematic Position:
Class: Chlorophyceae
Order: Siphonales
Family: Vaucheriaceae
Genus : Vaucheria
Occurrence of Vaucheria:
Vaucheria is represented by 54 species of which about 19 species are found in India. Vaucheria is found mostly in fresh water but about six species are marine and some are terrestrial found on moist soil.
The terrestrial species like V. sessilis and V. terrestris form green mats on moist soil in shady places in green houses. V. amphibia is amphibous. V. jonesii was reported by Prescott (1938) in winter ice in U.S.A. The common Indian species of Vaucheria are V. amphibia, V. geminata, V. polysperma, V. sessilis and V. uncinata etc.
Thallus:
The thallus is made of long, cylindrical well branched filaments. The filament is aseptate, coenocytic structure. The thallus is attached to substratum by means of branched rhizoids or branched holdfast called the haptera. The thallus of V. mayyanadensis is differentiated in subterranean branched rhizoidal system and an erect aerial system. The filaments are rough, interwoven and appear as dark green felt like structure.
Some species like V. debaryana show calcium carbonate incrustations. The branching may be lateral or dichotomous. The filaments are non-septate, the protoplasm with many nuclei is continuous along the entire length of thallus thus the coenocytic Vaucheria thallus makes siphonaceous structure (Fig. 1A, B). The septa formation occurs only during reproduction or in Gongrosira condition or for sealing of an injury.
The thallus structure is differentiated into cell wall and protoplasm. The cell wall of thallus is thin, weak and non-elastic. The cell wall is made of two layers, the outer layer is pectic and the inner layer is cellulosic. Inner to the cell wall there is thick layer of protoplasm. A very large central vacuole filled with cell sap runs from one end of the filament to another forming a continuous canal or siphon.
In peripheral part of protoplasm are present a large number of small oval or disc shaped chloroplasts which lack pyrenoids (Fig. 1 B). Christensen (1952) reported presence of pyrenoids in chromatophores.
The chromatophores in Vaucheria contain pigments, chlorophyll a, chlorophyll e, carotenoids and an unknown xanthophyll. The pigments in Vaucheria are like those of Xanthophyceae as chlorophyll b the characteristic pigment of Chlorophyceae is absent.
Many small nuclei lie in the cytoplasm inner to the layer of chloroplasts. The arrangement of nuclei with respect to chloroplasts is reversed at the time of zoospore formation. The cytoplasm also contains other membrane bound cell organelle such as mitochondria, small vesicles and food is stored in form of oil. The growth of filament is apical, the filament increases in length by apical growth of all the branches.
Nature of Thallus:
The thallus of Vaucheria is branched, non-septate and multinucleate structure which appears like single large cell but Vaucheria cannot be considered as single cell. As in multicellular forms mitotic divisions take place increasing the number of nuclei. The apical growth takes place. Hence the aseptate coenocytic structure of Vaucheria should be considered as acellular coenocyte.
Reproduction in Vaucheria:
Reproduction in Vaucheria takes place by vegetative, asexual and sexual methods.
(i) Vegetative Reproduction in Vaucheria:
The vegetative reproduction takes place by fragmentation. The thallus can break into small fragments due to mechanical injury or insect bites etc. A septum develops at the place of breaking to seal the injury. The broken fragment develops thick wall and later on develops into Vaucheria thallus.
(ii) Asexual Reproduction in Vaucheria:
The asexual reproduction takes place by formation of zoospores, aplanospores and akinetes
(a) By Zoospores:
The zoospores formation is the most common method of reproduction in aquatic species. In terrestrial species it takes place when the plants are flooded. Zoospore formation takes place in favourable seasons or can be induced if aquatic species are transferred from light to darkness or from running water to still water.
Zoospores are formed singly within elongated club shaped zoosporangium (Fig. 2A, B). The development of zoosporangium begins with a club shaped swelling at the tip of a side branch. A large number of nuclei and chloroplasts along with the cytoplasm move into it. A colourless protoplasmic region becomes visible at the base of cytoplasm and it is separated from rest of the cytoplasm of thallus.
Each separated protoplast secretes thin membrane and zoosporangium gets separated by a cross wall. Inside zoosporangium the vacuole decreases, the contents of sporangium become very dense and round off. The change takes place in relative position of chloroplasts and nuclei, the nuclei become peripheral and chloroplasts enter in inner layer of cytoplasm.
The entire protoplasm of the zoosporangium contracts to form oval zoospore. Opposite to each nucleus two flagella are produced making zoospore a multi-flagellate structure. A terminal aperture develops in zoosporangium by gelatinization of wall. The zoospore is liberated through aperture in morning hours (Fig. 2 C, D).
Each zoospore is large yellow green, oval structure. It has a central vacuole which has cell sap and may be traversed by cytoplasmic strands. The protoplasm outer to vacuole has many nuclei towards the walls and chromatophores towards vacuoles. Two flagella arise opposite to each nucleus. This part of cytoplasm can be regarded equivalent to one zoospore.
Fritsch (1948) regarded this kind of zoospore as compound zoospore or synzoospore as a number of biflagellate zoospores have failed to separate from one another.
According to Greenwood, Manton and Clarke (1957) the flagella of a pair are heterokontic and whiplash type. The shorter flagellum of each pair is directed towards the anterior end of the zoospore. The flagellar bases are united together in pairs and are firmly attached to the tip of nuclei.
According to Greenwood et. al (1957), there is large anterior vacuole and small ones in the posterior region of the zoospores. Mitochondria are present in the peripheral layer of cytoplasm. Fat bodies and plastids are present in the cytoplasm. Chlorophyll has also been reported from the zoospores.
The zoospores swim in water for 5-15 minutes and germinate without undergoing any significant period of rest. The zoospores get attached to the substratum, withdraw flagella and secrete thin walls (Fig. 2 E, F). The chromatophores move outwards and nuclei inwards as in vegetative condition.
The two tube like outgrowths develop in opposite directions. One of the two outgrowths elongates, branches to form colourless lobed holdfast and the other outgrowth forms yellow-green tubular coenocytic filament (Fig. 2 G, H).
(b) By Aplanospores:
Aplanospores are commonly observed in species. V. geminata, V. uncinata and in marine species V. pitoboloides. The aplanospores are generally formed by terrestrial species.
Aquatic species form aplanspores under unfavorable condition of drought. The aplanospores are non-motile asexual spores formed in special structures called aplanosporangia (Fig. 3 A-C). The aplanospores are produced singly in cells at the terminal end of the short lateral or terminal branch.
The protoplasm of aplanosporangium gets metamorphosed into single multinucleate aplanospore which is thin walled. In V. germinata aplanospores are oval and are liberated from apical pore formed by gelatinization.
In V. uncinata aplanospores are spherical and are liberated by rupture of the sporangial wall. The formation and structure of aplanospores and zoospores is similar except that the zoospores lack flagella. The aplanospores soon after liberation germinate into new thalli (Fig. 3D).
(c) By Akinetes:
Akinetes are thick walled structures formed during unfavorable conditions like drought, and low temperature. The akinetes have been commonly observed in V. geminata, V. megaspora and V. uncinata.
The akinetes are formed on the terminal part of lateral branches where protoplasm migrates to the tips followed by cross-wall formation (Fig. 4). These multinucleate, thick walled segments are called akinetes or hypnospores.
The akinetes by successive divisions may form numerous thin walled bodies called cysts. When many akinetes remain attached to the parent thallus, the thallus gives the appearance of another alga Gongrosira.
Hence this stage of Vaucheria is called Gongrosira stage. During favourable conditions the akinetes and cysts develop into new thalli. Randhawa (1939) has reported that in V. uncinata the submerged parts of thallus develop sex organs whereas exposed parts of thallus form brick shaped akinetes.
(iii) Sexual Reproduction in Vaucheria:
In Vaucheria sexual reproduction is of advanced oogamous type. The male and female sex organs are antheridia and oogonia, respectively.
Majority of the freshwater species are monoecious or homothallic while some species like V dichotoma, V. litorea and V. mayyanadensis are dioecious or heterothallic. There are different types of arrangement of antheridia and oogonia in homothallic species. The position, structure and shape of antheridia are of taxonomic importance in Vaucheria.
The common patterns of arrangement of sex organs are as follows:
(a) Antheridia and oogonia develop close to each other on the filament at intervals (Fig. 5 A-C).
(b) The antheridia and oogonia are borne on special side branches with a terminal antheridium and a number of lateral oogonia (Fig. 5D).
In V. hamata the reproductive branches bear a median terminal antheridium and two oogonia, one on either side of antheridium.
In V. geminata and V. terrestris the sex organs are produced at the ends of the lateral branches with a terminal antheridium and a group of oogonia (Fig. 5D). The sex organs are unilateral when they are arranged on one side of the filament or bilateral when they are on both sides of the filament.
(c) Antheridia and oogonia are borne on adjacent branches (Fig. 5E).
Structure and Development of Antheridium:
The mature antheridia may be cylindrical, tubular, straight or strongly curved. The antheridium is separated from main filament by a septum. The antheridia can be sessile (without stalk) arising directly from main branch e.g., V. civersa. The antheridia may be placed high on the branch the antheridia are situated on androphore V. synandra.
The young antheridium is usually green in colour. It contains cytoplasm, nuclei and chloroplasts. The mature antheridia are yellow and contain many spindle shaped antherozoids. The antherozoids are liberated through a terminal pore e.g., V. aversa or through many pores e.g., V. debaryana
In monoecious species the antheridium arises as a small bulging or lateral outgrowth along with or before the oogonium development (Fig. 6A). Many nuclei along with cytoplasm enter into it and it gets cut off from the lower part forming a septum.
The antheridium grows and becomes high curved structured, its upper part is main antheridium and the lower part is stalk. The nuclei of antheridium get surrounded by cytoplasm and develop into biflagellate, yellow coloured antherozoids The antherozoids are liberated from the tip of antheridium through apical pore shortly before day break (Fig. 6D-1).
Structure and Development of Oogonium:
The oogonium development starts with accumulation of colourless multinucleate mass of cytoplasm near the base of antheridial branch. This accumulated cytoplasm has been termed as “wanderplasm”. The wanderplasm enters into the outgrowth or bulging of the main filament. This outgrowth is called as oogonial initial.
Large amount of cytoplasm and nuclei enter into oogonia, making it a large globular structure called as oogonium (Fig. 6 B-E). As the oogonium matures, it gets separated from main branch by the development of septum at its base. The mature oogonium is uninucleate structure. The nucleus of oogonium with protoplasm develops into a single egg.
There are three hypothesis regarding the fate of extra nuclei of oogonium of Vaucheria:
(a) According to Oltmanns (1895) accept a single nucleus which forms female nucleus, all other nuclei migrate back into the filament. This was supported by Heidinger (1908) and Couch (1932).
(b) According to Davis (1904), the single nucleus forms the egg and all other nuclei degenerate.
(c) According to Brehens (1890) all nuclei fuse to form a single nucleus.
The mature oogonia are globose, obovoid, hemispherical or pyriform in shape. The oogonia may be sessile or stalked structure. The protoplast of oogonium is separated from main filament by- septum formation.
The entire protoplasm with single nucleus makes a central spherical mass called as oosphere or ovum. In mature oogonium a distinct vertical or oblique beak develops in apical part. Opposite to beak develops a colourless receptive spot. A pore develops just opposite to receptive spot (Fig. 6 F).
Fertilization:
The oogonium secretes a gelatinous drop through a pore near the beak. A large number of liberated antherozoids stick to the drop. Many antherozoids push into the oogonium. The antherozoids strike violently, fall back and push forward again and fall back. Only one antherozoid enters into the oogonium.
After its entry the membrane develops at the pore to stop the further entry of antherozoids. The male nucleus increases in size and fuses with the egg nucleus to make diploid zygote. The zygote secretes a thick 3-7 layered wall and is now called as oospore (Fig. 6 G-I). The chromatophores degenerate and lie in the centre of the cell.
Germination of oospore:
The oospore undergoes a period of rest before germination. During favourable season the oogonial wall disintegrates and the oospore is liberated. The oospore germinates directly into new filaments.
Although the exact stage at which the reduction division takes place in Vaucheria is not clear, it is believed that reduction division occurs in first nuclear division in the germinating oospore (Fig. 7 A-D). The oospore germinates to make haploid thallus of Vaucheria.
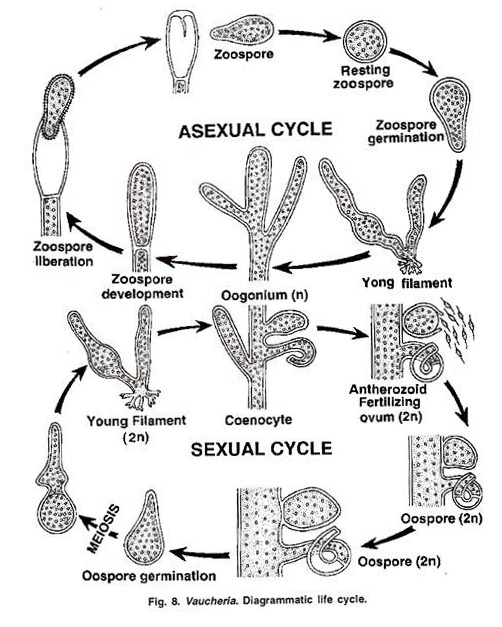
Life Cycle of Vaucheria:
According to Williams, Hanatsche and Gross the life cycle of Vaucheria is haplontic, the oospore being the only diploid structure in life cycle (Figs. 8, 9). Vaucheria thallus is haploid. It is aseptate, branched, tubular and coenocytic structure.
Vegetative re-production takes place by fragmentation. Asexual reproduction takes place by zoospore in aquatic species and by aplanospores in terrestrial species.
The zoospore is large multi flagellate structure and is supposed to be compound:
Zoospore or Synzoospore.
The sexual reproduction is advanced oogoinous type, the male and female sex organs are antheridia and oogonia. Most of the species are homothallic, some are heterothallic. After fertilization, a diploid zygote is formed which converts into oospore and undergoes a period of res The reduction division takes place in oospore during germination and an haploid thallus is formed (Fig. 8, 9).
Systematic Position and Affinities of Vaucheria:
The position of Vaucheria in algae has always been debatable. Fritsch (1935) placed it in the order Siphonales of the class Chlorophyceae, it was also supported by Iyengar (1951). Chadefaud transferred it to Xanthophyceae and Smith (1959) placed it in order Heterosiphonales of the class Xanthophyceae. This view was supported by Chapman (1962), Taylor, Prescott (1969) and Morris (1968).
A. Affinities of Vaucheria with Xanthophyceae:
(i) Siphonaceous, acellular thallus.
(ii) Predominance of carotenoids, over chlorophylls. Absence of chlorophyll b from Vaucheria which is a characteristic pigment of Chlorophyceae.
(iii) Chloroplasts without pyrenoids.
(iv) Reserve food material is oil, instead of starch.
(v) In Vaucheria antherozoids flagellation is heterokontic. There are two lateral, unequal flagella. The anterior flagellum is tinsel type and the posterior as whiplash type.
B. Affinities of Vaucheria with Chlorophyceae:
(i) Multinucleate, aseptate, coenocytic thallus.
(ii) Sexual reproduction being advanced oogamous type.
C. Affinities of Vaucheria with Oomycetes (Fungi):
(i) The development of the sex organs in Vaucheria show striking resemblance to some members of Oomycetes.
(ii) Coenocytic nature of thallus is like that of Saprolegniaceae.
These features along with heterokontic flagella in antherozoids suggest that lower fungi could have been derived from Vaucheria like ancestors.