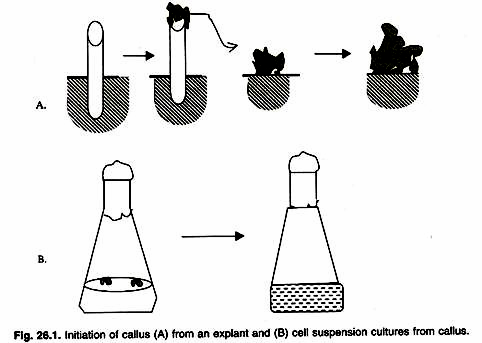
(A) Physical Properties of Soils:
Physical properties of the soil can be discussed under the following heads:
(1) Soil separates and texture,
(2) Structure of soil,
(3) Weight and soil density,
(4) Porosity of soil,
(5) Permeability of soil,
(6) Soil colour,
(7) Temperature of soil, and
(8) Soil Plasticity, Compressibility and Erodibility.
1. Soil Separates and Soil Textures:
Mineral fraction of soil consists of particles of various sizes. According to their size, soil particles are grouped into the following types (Table 23.1).
The particle sizes of above groups are suggested by International Society of Soil Science. In India, international system of particle differentiation is commonly followed. The particle types are generally called ‘soil separates’ or ‘soil fractions’. Amount of soil separates is determined by a process known as mechanical analysis. In this process, soil sample is crushed and screened through a 2 mm round hole sieve. The screened soil is then homogeneously dispersed in water and allowed to settle.
In suspension, particles of largest dimensions will settle first and those of smaller dimensions will settle afterwards. Individual soil separates are identified on the basis of their respective diameter ranges. Soil separates (sand, silt and clay) differ not only in their sizes but also in their bearing on some of the important factors affecting plant growth, such as, soil aeration, workability, movement and availability of water and nutrients.
Important characteristics of different soil separates are as follows:
Sand:
This fraction of soil consists of loose and friable particles of 2.203—.02 mm diameter. Sand particles can be seen by unaided eye. These particles, although inactive, constitute the framework of the soil. They play less important role in physicochemical activities. When coated with clay, these sand particles take very active part in chemical reactions. Sands increase the size of pore spaces between soil particles and thus, facilitate the movement of air and water in the soil.
Silt:
It consists of soil particles of intermediate sizes between sand and clay (diam range .02—.002 mm). Silt, when wet, feels plastic but in dry state feels like flour or talcum. Coarse silt shows little physicochemical activities but finer grades play important role in some chemical processes. Silty soil has got larger exposed surface area than the sandy soil. Silty soils contain sufficient quantities of nutrients, both organic and inorganic. That is why they are very fertile. Soils rich in silt possess high water holding capacity. Such soils are good for agriculture.
Clay:
This soil fraction contains smaller particles than silt(below .002 mm diameter) which exhibit plasticity and smoothness when wet and hardness when dry. Owing to their smallest size and colloidal nature, the clay particles expose extremely large surface area. They take very active part in physicochemical reactions of the soil. Clay soils have fine pores, poor drainage and aeration and thus they have highest water holding capacity. The clay acts as store house for water and nutrients.
Some soils are fine, while others are coarse. It is so because of the fact that the relative percentage of sand, silt and clay differ from soil to soil. The relative percentage of soil separates of a given soil is referred to as soil texture. Texture of soil for a given horizon is almost a permanent character, because it remains unchanged over a long period of time.
The relative percentages of soil separates of average samples are almost infinite in possible combinations. It is, therefore, necessary to establish limits of variations among soil fractions so as to group them into textural classes. The common textural classes, as recognized by USDA (U.S. Department of Agriculture) are given in the following table. These classes are recognized on the basis of relative percentage of separates; sand, silt and clay (Table 23.2).
This chart is adapted from fraction system of U.S.D.A. If relative percentages of soil separates are known, the soil can be given textural name. For this purpose equilateral triangles are used. The most widely used Equilateral triangles are international equilateral triangle and the one used by USDA (Figs. 23.1, 23.2). These consist of three angles and its area is divided into twelve groups representing twelve different textural classes. Each group covers definite range of percentages of sand, silt, and clay. In the triangles, left side line represents the clay %, right side line represents percentage of silt and base represents percentage of sand.
Each arm of the triangle is divided into ten divisions representing soil separate’s percentage. These divisions are further divided into ten small divisions; each small division represents one per cent of soil separate. The percentages of sand, silt, and clay obtained after mechanical analysis of the given soil are read on the equilateral triangle.
In using the diagram as indicated the percentages of silt and clay should be located on silt and clay lines respectively. The line in case of silt is then projected inward parallel to clay side of the triangle and in case of clay it should be projected parallel to the sand side. The three lines; one representing sand percentage, other representing silt percentage and the third clay percentage meet at a point in the triangle. The compartment in which the point falls indicates textural name for the given soil sample. The knowledge of soil texture is of great help in the classification of soil and in determination of degree of weathering of rock.
2. Structure of Soil:
Sand, silt and clay are found in aggregated form. Arrangement of these soil particles on certain defined patterns is called soil structure. The natural aggregates of soil particles are clod peds whereas an artificially formed soil mass is called clod. Ped differs from fragment because the latter refers to the broken ped. Ped differs from concretion in the sense that the latter is formedinthe soil by precipitation of salts dissolved in percolating water.
Soil structure also reveals the colour, texture and chemical composition of soil aggregates. Soil structure is influenced by air moisture, organic matter, micro-organisms and root growth. When many particles or peds are aggregated into cluster, a compound particle is formed.
Soil Structure is described under the following three categories:
A. Type:
This indicates the shapes or forms and arrangement of peds. Peds may be of various shapes, such as granular, crumb, angular blocky, sub angular blocky, platy and prismatic (Fig. 23.3). Different types of peds and their properties are describedin (Table 23.3).
B. Size Class:
These are as follows:
(i) Very fine or very thin
(ii) Fine or thin
(iii) Medium
(iv) Coarse or thick
(v) Very coarse or very thick
C. Grade:
This indicates the degree of distinctness of peds.
It is described under the following four categories:
(i) Structure less:
Peds not distinct, i.e., cement or sand like condition.
(ii) Weak:
Peds distinct and rarely durable.
(iii) Moderate:
Peds moderately well developed, fairly durable and distinct.
(iv) Strong:
Peds well developed, quite durable and distinct.
3. Density and Soil Weight:
Density of soil is the mass per unit volume. It is expressed in terms of gm per cubic centimeter. Average density of the soil is 2.65 gms per cubic centimeter. Density of soil varies greatly depending upon the degree of weathering.
For this reason soil density is expressed in two generally accepted forms:
(i) Particle density or true density; and
(ii) Bulk density.
(i) Particle density:
Density of solid portion of soil is called particle density. It is sum total of densities of individual organic and inorganic particles. Average particle density of organic soil varies from 1.2 to 1.7 gms per ml. and that of inorganic fraction varies from 2.6 to 2 78 gms/ ml. Particle density may be calculated as: weight of solids / volume of soils. Particle density divided by density of water gives the specific gravity or relative weight number. Specific gravity of soil particles = Particle density /density of water.
(ii) Bulk density or apparent density:
Dry weight of unit volume of soil inclusive of pore spaces IS called bulk density. It is expressed in terms of gm per ml or lbs per cubic foot. It is lesser than the particle density of the soil. Bulk density of soil may be calculated as: weight of soil/ volume of soil.
Bulk density of the soil divided by density of water gives volume weight or apparent specific gravity of soil. Bulk density of soil changes with the change in total pore space present in the soil and it gives a good estimate of the porosity of soil. Average density of soil in bulk is 1 5 gm/ml. Organic soils have low bulk density as compared to mineral soils. Soil weight varies in relation to textural classes. Average weight of loam or sandy soil is 80—110 pounds/cubic foot but that of clay ranges between 70 and 100 pounds/cubic foot.
4. Porosity of Soil:
The spaces occupied by air and water between particles in a given volume of soil are called pore spaces. The percentage of soil volume occupied by pore space or by the interstitial spaces is called porosity of the soil. It depends upon the texture, structure, compactness and organic content of the soil. Porosity of the soil increases with the increase in the percentage of organic matter in the soil. Porosity of soil also decreases as the soil particles become much smaller in their dimension because of decrease in pore spaces.
It also decreases with depth of the soil. The pore spaces are responsible for better plant growth because they contain enough air and moisture. Percentage of solids in soils can be determined by comparing bulk density and particle density and multiplying by hundred.
Depending upon the size pore spaces fall into two categories.
These are:
(1) Micro-pore spaces (capillary pore spaces)
(2) Macro-pore spapes (non-capillary pore spaces)
Capillary pore spaces can hold more water and restrict the free movement of water and air in soil to a considerable extent, whereas macro-pore spaces have little water holding capacity and allow free movement of moisture and air in the soil under normal conditions.
5. Permeability of Soil:
The characteristic of soil that determines the movement of water through pore spaces is referred to as soil permeability. Soil permeability, because it is directly dependent on the pore size, will be higher for the soil with large number of macro-pore spaces than that for compact soil with a large number of micro-pore spaces (capillary spaces). Permeability of soil also varies with moisture status and usually decreases with the gradual desiccation of soil. In the arid regions, groundwater moves upwardly through capillary action and bring sodium, potassium and calcium salts with it in dissolved state on the surface of soil. The water evaporates and inorganic salts precipitate on the surface of the soil. As a result of this, the soil becomes less permeable and the productive capacity of soil is reduced.
6. Soil Colour:
Soils exhibit a variety of colours. Soil colour may be inherited from the parental material (Le., lithochromic) or sometimes it may be due to soil forming processes {acquired or genetic colour). The variations in the soil colour are due to organic substances, iron compounds, silica, lime and other inorganic compounds.
The organic substances impart black or dark greyish-black colour to the soil. Iron compounds are responsible for brown, red and yellow colours of soils. Iron oxides in combination with organic substances impart brown colour which is most common soil colour. Silica, lime and some other inorganic compounds give light white and grey tinges to the soil.
Soil colour influences greatly the soil temperature. The dark coloured soils absorb heat mort readily than light coloured soils. The work of Ramdas, L.A. and David, R.K. (1936) at Poona showed that black cotton soil absorbed 86% of the total solar radiations falling on the soil surface as against 40% by the grey alluvial soil. Soil colour is used as an important criterion for description and classification of soil. Many soils are named after their prominent colours, such as black cotton soil, red-yellow latosol, grey hydromorphic soils and so on.
7. Soil Temperature:
The chief sources of soil heat are solar radiations and heat generated in the decomposition of dead organic matters in the soil and heat formed in the interior of earth. The soil temperature greatly affects the physico-chemical and biological processes of the soil. Temperature of soil depends upon the temperature of atmospheric air and on moisture content. It is controlled by climate, colour of soil, slope, and altitude of the land and also by vegetational cover of the soil.
The average annual temperature of soil is generally higher than that of its surrounding atmosphere. Ramdas and M.S. Katti (1934) recorded surface temperature of black cotton soil as high as 165″? at Poona (Maharashtra), India. Surface temperature of soil shows considerable fluctuations but soil temperature below certain depth remains more or less constant and is not affected by diurnal or regional temperature changes.
Studies made by Leather at Pusa Research Institute in Bihar (India) showed that diurnal temperature difference at the level 12 inches below the soil surface was only 1°C and at a depth of 24″ it seldom exceeded 0.1 °C. At the depth of 3 or 4 feet, the temperature remains almost constant.
8. Soil Plasticity, Compressibility and Erodibility:
Soil plasticity is a property that enables the moist soil to change shape when some force is applied over it and to retain this shape even after the removal of the force from it. The plasticity of soil depends on the cohesion and adhesion of soil materials. Cohesion refers to the attraction of substances of like characteristics, such as, that of one water molecule for another. Adhesion refers to the attraction of substances of unlike characteristics. Soil consistency depends on the texture and amount of inorganic and organic colloids, structure and moisture contents of soil.
Compressibility:
It refers to the tendency of soil to consolidate or decrease in volume. The compressibility is partly a function of elastic nature of soil particles and is directly related to settlement of structures. With the decrease in the moisture contents soils gradually tend to become less sticky and less plastic and finally they become hard and coherent. Plastic soils have great cohesion force. It is only because of cohesion property the moist clay soils frequently develop cracks when they become dried (Fig. 23.4). Coarse materials such as gravels and sands have low compressibility and the settlement is considerably less in these materials as compared to highly compressible fine grained organic soils.
Erodibility:
It refers to the ease with which soil materials can be removed by wind or water. Easily eroded materials include unprotected silt, sand and other loosely consolidated materials, Cohesive soils (with more than 20% clay) and naturally cemented soils are not easily removed from its place by wind or water and, therefore, have a low erosion factor.
(B) Chemical Properties of Soils:
Chemical properties of soils can be described under the following heads:
(1) Inorganic matters of soil,
(2) Organic matters in soil,
(3) Colloidal properties of soil particles, and
(4) Soil reactions and Buffering action,
(i) Acidic soils,
(ii) Basic soils,
1. Inorganic Matters of Soil:
From the accounts given in the description of weathering process it is clear that compounds of aluminium, silicon, calcium, magnesium, iron, potassium and sodium are chief inorganic constituents of soils. Besides these, the soils also contain small quantities of several other inorganic compounds, such as those of boron, magnesium, copper, zinc, molybdenum, cobalt, iodine, fluorine etc. The amounts of these chemicals vary in soils of different places. Chemical composition of soil of one horizon differs greatly from the composition of soil in the other horizon.
2. Organic Matters in Soil:
Organic component of the soil consists of substances of organic origin; living and dead. In sandy soil of arid zone, it is found in very poor quantity (one or less than one per cent) but in peaty soil, it may be as high as 90%. When the plants and animals die, their dead remains are subjected to decomposition.
As a result of decomposition a number of different organic products or compounds are formed from the original residues. In the course of decomposition, the original materials are converted into dark coloured organic complexes, called humus. Sometimes living micro-organisms add sufficient amount of organic matters in soil in the form of metabolic wastes.
Chemists have been attempting to unravel the details of humus composition since the earliest days of soil science, and have got much success but more is yet to be discovered. In terms of specific elements, the organic component of soil contains compounds of carbon, hydrogen, oxygen, phosphorus, nitrogen, sulphur and small amount of other elements also. Only small fraction of total organic matter is soluble in water but majority of them are soluble in alkali solution.
Chemically humus contains the following organic molecules:
A. Amino Acids:
i. Glutamic acid
ii. Alanine
iii. Valine
iv. Proline
v. Cystine
vi. Phenyl alanine
B. Proteins:
(I) Purines
(i) Guanine
(ii) Adenine
(II) Pyramidines
(i) Cytosine
(ii) Thymine
(iii) Uracil
C. Aromatic Molecules:
D. Uronic Acids:
(i) Glucuronic acid
(ii) Galacturonic acid
(iii) Lactic acid
E. Aliphatic Acids:
(i) Acetic acid
(ii) Formic acid
(iii) Succinic acid
F. Aminosugars:
(i)Glucosamine
(ii) N. Acetylglucosamine
G. Pentose Sugars:
(i) Xylose
(ii) Arabinose
(iii) Ribose
H. Hexose Sugars:
(i) Glucose
(ii) Galactose
(iii) Manose
I. Sugar Alcohols:
(i) Inosital
(ii) Mannitol
J. Methyl Sugars:
(i) Rhamnose
(ii) Fucose
(iii) 2-0, methyl D-xylose
(iv) 2-0, methyl D-arabinose
Besides these compounds locked up in the humus fraction, the soil also contains fats, oils, waxes, resins, tannin, lignin and some pigments.
Waksman and Stevans have proposed the following method for separation of different organic compounds present in the soil:
Another modified method for separation of the various organic compounds from the soil is as follows:
The fractions are not pure chemical compounds but are in the form of mixtures of several substances. They are found in colloidal state in the soil.
3. Colloidal Properties of Soil Particles:
There are two types of substances namely crystalloids and colloids:
Crystalloids are those crystalline solid substances which form true solution on being mixed with other substances. In true solution, crystal particles cannot be seen with the help of microscope.
The word colloid first coined by Grahm (1849) is derived from Greek words kolla meaning glue and eoids meaning appearance, i.e., glue like in appearance. Colloid is really speaking amorphous state of the substances which do not form true solution if mixed with other substances. The particles of colloidal substances float in the solvent in suspension state but do not tend to settle at the bottom. Colloids are not found in ionic or molecular form but are found in aggregates of atoms or molecules.
Colloidal system or suspension contains two phases which are:
(i) Dispersion phase, i.e., medium in which the particles are suspended, and
(ii) Dispersed phase, i.e., suspended particles.
Colloidal suspension may be of different kinds, such as:
(1) Suspension of liquid, in liquid, as milk (fats in water).
(2) Suspension of solid in liquid as India ink (or clay suspension in water).
(3) Suspension of solid in gas, as smoke (coal particles suspended in air).
(4) Suspension of liquid in gas, e.g., cloud and fogs in atmosphere.
The commonest colloids are those which remain suspended in a liquid medium:
If the colloidal suspension exhibits properties of fluid, it is called sol, but sometimes sols exhibit solid like behaviour and form solid or nearly so. This condition is called gel. Some sols form reversible gel while the others form irreversible gel.
Some of the important properties of colloids in general are as follows:
1. Particle size:
Crystalloids and colloids differ from each other in their size range Particles of crystalloid in true solution are 0.2 to 1 mµ (millimicron) while those of colloids in suspension are 1 to 200 mµ.
2. Adsorption:
Because the colloidal particles of dispersed phase are very small, they have got large exposed surface areas. Owing to their large exposed surface areas, these colloidal particles show great adsorptive capacity. In adsorption, particles of particular substances come to lie on the surface of colloids and they do not enter deep in the colloidal particles.
3. Electrical properties:
The electrically charged colloids are termed as micelles. Colloids have some electrical charge on them. They may be charged either positively or negatively. Colloidal particles of one electrical charge have tendency to attract colloids of opposite charge. In the soil clay particles are negatively charged, thus they attract cations (+ charged ions).
Colloid particles differ from electrolytes in the fact that when electric current is passed in the colloidal suspension, all the colloidal particles are attracted towards one electrode or the other depending upon the nature of charge they carry on them. This phenomenon is called electrophoresis.
The electrolytes, when dissolved in solvent dissociate into two types of ions among which half will bear positive charge (cations) and remaining half will bear negative charge (anions) When electric current is passed in the solution of electrolyte all the positively charged ions will accumulate on negative pole and remaining negatively charged ions will collect on positive pole.
4. Coagulation or flocculation of colloidal particles:
Colloidal particles in the suspension can be coagulated either by heating or by adding some substances which contain opposite charged ions. When substances carrying positive ions are added in suspension containing negatively charged colloid particles, ions will move and accumulate on the surface of colloids carrying opposite charge. Finally a stage comes when colloidal particles cannot attract more opposite charged ions This is called isoelectric point As a result of ion accumulation on their surface, the colloids first become large and heavier and finally they tend to settle at the bottom in floccules. This process is known as flocculation.
5. Tyndal phenomenon:
Colloidal particles in suspension can be seen when a strong beam of light is passed through suspension and observer looks it from the place at right angle to the path of light. The colloidal particles become visible as strongly illuminated particles and they appear bigger than normal size. This phenomenon is known as “Tyndal effect”.
6. Brownian movement:
Colloidal particles when suspended in dispersion medium show a characteristic continuous zig-zag motion, called Brownian movement. This type of movement was first observed by English botanist Robert Brown, hence it is called Brownian movement. The movement is exhibited because of characteristic collision of one particle with others. This prevents the particles from settling down.
7. Dialysis:
Because colloidal particles in suspension are larger than the particles of crystalloid in true solution and are larger than the diameter of pores of porous membranes, e.g., parchment membrane, they are not allowed to be filtered down and are retained the membrane Thus, they can be separated in pure state from the mixture of crystalloids and colloids by filtration process. This separation process is known as dialysis.
Colloidal Fraction of Soil:
There are two types of colloids in the soil. These are:
(1) Mineral colloids or clay colloids, and
(2) Organic or humus colloids.
These two colloidal fractions of soil are very intimate to each other and it is very difficult to separate them. The inorganic colloids occur as very fine particles and organic colloids occur in the form of humus particles. The soil colloid particles show almost all the characteristics of typical colloidal system, i.e., adsorption, Tyndal effect, Brownian movement, coagulation, electrophoresis, dialysis etc.
1. Clay colloids:
As regards the size, clay fraction of soil contains both non-colloidal and colloidal particles. Some clay particles may be as large as .002 mm in diameter but some may be smaller than normal colloid size (normal size of colloid particle is from 1 to 200 mµ). The clay particles are formed mainly of silica, alumina, iron and combined water. Colloidal clay may also contain rich accumulation of plant nutrients.
Early researchers of soil science have described clay colloids as spherical particles and their sizes were mentioned in terms of their diameters, but recent electron micrographs reveal that particles occur in layers or plates and each c ay particle appears as if it is composed of a large number of plate-like units. These units or flakes of clay are held together by a force of attraction. The plate-like clay particles expose large surface area on which moisture and cations (+ ions) are held. The finer the clay particles the greater will be the percentage of hygroscopic moisture.
If clay is suspended in distilled water, shaken, and then a little NH4OH is added to suspension and allowed to settle, after a few minutes large particles settle down but finer particles remain in suspended state. When a little limewater is added to suspension, fine suspended particles increase in size and form small floccules which have a tendency to settle down. Thus, finer clay particles show flocculation property. Now if some acid is added, the floccules are broken and the clay particles will return to their normal size. This process is known as deflocculating.
The clay particles are negatively charged, hence they can hold thousands of positively charged ions of mineral nutrients on their surfaces. The clay colloids are lyophilic (water loving). So, they are important from the standpoint of the adsorption of large quantity of water (perhaps, 5-10 layers of water molecules are held on the surface of clay colloid).
2. Organic colloids or humus colloids:
Organic colloids in the soil are chiefly due to presence of humus. The humus contains 8% each of lignin, protein, polyuronides (sugars and uronic acid complex). Organic colloids may be present in appreciable proportion in the soils. In sandy soil, it forms minor part of colloids. In peaty soil, organic colloids may be more than 50%. These colloids show adsorptive capacity many times greater than clay colloids. Organic colloids are negatively charged like clay colloids. Addition of organic colloids to the sandy soil increases temporarily its moisture and nutrient retaining capacity.
Cation Exchange:
Since the soil colloids (clay and organic colloids) have negative charges on them, they attract and hold positive ions (cations). When cations are added to the soils such as Ca++ in the form of lime, K+ ions in the form of potassium fertilizer, and NH++ in the form of ammonium fertilizer, the adsorption of cations will take place on the surface of colloid micelle and this will be accompanied by release of one or more ions held by colloid micelle.
This is known as cationic exchange. For example, suppose that colloid micelle has one half of its capacity satisfied with Ca++ ions, one quarter with K+ ion and remaining one quarter with H+ ions. Now the colloid is treated with KCl solution. The K+ ions will first replace Ca++ ions and then H+ ions. The Ca++ and H+ ions will combine with CI– ions of KCl and will form CaCl2 and HCl respectively.
The cation exchange in the soil may take place between:
(1) Cations present in the soil solutions and those already present on surface of soil colloids
(2) Cations released by plant roots and those present on the surface of soil colloids, and
(3) Cations present on the surface of two clay crystals either two organic colloids or an organic colloid and a clay colloid.
Exchange reaction is very quick and reversible and the exchange of ions continues till equilibrium is attained. All cations are not adsorbed with equal ease. Some are easily adsorbed while others are replaced with difficulty. Divalent cations are more effective than the monovalent ones. Hydrogen is exception because it is held by colloids most tenaciously and it IS most powerful replacer of cations.
Replacing capacities of some cations are compared here
H+ > Ca++ > K+ > Na+> Na+
The number of cations adsorbed per unit weight of one hundred grams dry soil is called cation exchange capacity. More scientifically, cation exchange capacity of soil is the sum total of exchangeable cations adsorbed per unit weight of one hundred gms of dry soil.
Factors which are responsible for cation exchange or base exchange are as follows:
(1) Relative concentration and number of cations present in the soil,
(2) Replacing capacity of the ions, and
(3) Number of charges on the ions.
Anion Exchange:
Soils rich in organic colloids show anion exchange also. In this process, negatively charged ions held by colloids are replaced by OH–, H2PO4–, SO4—, and NO3– ions. The relative order of exchange is
OH– > H2PO4–, > SO4— > NO3–
Among these anions, exchange of PO4—, ions is most important. SO4— and NO— are not retained in the soil for long period of time, hence not available for anionic exchange. Laterite soils have high adsorptive and fixation capacity for PO4— than black soils.
Anionic and cationic exchange reactions are important in agriculture. Several soil scientists have shown that the capacity of soil to exchange cations is the best index of soil fertility. The predominance of desirable ions in the exchange complex brings about good physical cations and favorably influences the microbial activities in the soil, such as ammonification nitrification, etc. The knowledge of cation and anion exchange is of great help in reclaiming acidic and saline or alkaline soils.
4. Soil Reaction:
Many chemical properties of soils centre round the soil reaction. As regards their nature, some soils are neutral, some are acidic and some basic. The acidity, alkalinity and neutrality of soils are described in terms of hydrogen ion concentrations or pH values. In order to understand soil reaction, the knowledge of pH is very necessary.
It can be understood in the following ways:
Water dissociates into H+ ion and OH– ion. Hence the ionic constant of water can be represented as follows:
Ionic constant of water = [H+][OH–]/[H2O]. But the rate of dissociation of water is so slow that ionization constant of water can be expressed simply as product of concentration of H+ and OH– ions, thus ionization constant of water Kw = [H+][OH–]. Concentration of H+ and OH– ions are expressed in terms of equivalents per litre. Only one molecule in ten million water molecules is in dissociated condition.
At neutrality, H+ concentration is 0.0000001 or 10-7 gm of hydrogen per litre solution. The ionization constant of water is 10-14 at 25°C and thus in any aqueous system products of H+ and OH– ion concentration is 10-14.
Now, the above equation can be written as:
10-14 = [H+][OH–]
This can also be represented in the following way by dividing both sides in one and taking logarithms.
The value of log 1/[H+] and log 1/[OH–] are generally pH and poH respectively. These pH and pOH are indices of the acidity and alkalinity respectively. Thus, pH can be defined as negative logarithms of the H+ ion concentration. When the system is neutral, pH will be equal to pOH and when Kw is 10-14, the value of pH and pOH at neutral point will be 7 for each. When pH value is less than 7, it is acidic. The pH value above 7 indicates alkalinity. Ifina system, hydrogen ion concentration is 1/.000001 or 000001 gm/litre, the pH value will be 6.
It is more clear from the following calculation:
Thus at pH value of 6, the H+ ion concentration is increased 10 times than the H+ concentration at pH value of 7. Like this, at every lower pint H+ ion concentration will increase by a multiple of 10. As the product of H+ ion and OH– ion concentrations at neutrality is always 10-14, the pOH can be determined from pH value. Suppose, pH value of a solution is 6, the pOH value will be 8 (10-6 +10-8 = 10-14) pH scale is divided into 14 divisions or pH units from 1 to 14. Soil with pH value of 7 is neutral, that below pH 7 is acidic and that with pH value above 7 is alkaline.
From the pH value intensity of acidity in the soil is expressed but these values are not the measure of total acidity because they do not indicate the reserved acidity or relative acidity. For example, there are two soil samples which have similar pH values but they require different quantities of lime for neutralization. It means that the quantities of acids are different in the given weight of above two soils.
Buffer Action:
It refers to the resistance to change in pH of a system. Such solutions as are reasonably permanent in pH value even after addition of some alkali or acid to them are called solution with reserved acidity or alkalinity or more often “buffer solutions”. Suppose, a certain amount of acid is added to distilled water, the resulting solution will show acidic reaction and that will have a pH below 7, but if the same quantity of acid is added to a neutral soil suspension there would be very minor change in pH. This property of soil to resist a change in pH is called “buffer action”.
Buffer solutions are usually formed of a mixture of salt of weak acid and acid itself in various proportions, as for example, a mixture of sodium acetate and acetic acid if added to water will result in a buffer solution. In the mixture solution we have mainly sodium ions and acetate ions. In water there will be some H+ and OH– ions. In buffer solution, acetate ions are in excess, owing to presence of well ionised sodium acetate. If H+ ions are added to this solutions they will combine with acetate ions to give acetic acid of low ionisation power.
Hence, there will be a little increase in pH. The addition of acid to buffer solution then makes little difference in the pH value.
Buffering in Soil:
Soils should have good buffering capacity Therefore, it is necessary to add considerably large amount of acids or alkalis in order to bring about any change in the original pH of soil. Buffering action is due to presence of large quantity of weak acids and their salts in the soil. Phosphates, carbonates, bicarbonates and other salts of weak inorganic acids and corresponding acids themselves are important buffering agents in the soils. Besides these, colloids associated with cations are important buffering agents. The buffering action of soil is directly governed by the amount and nature of clay and organic or humus colloids present in it.
Buffering action of soil is important in agriculture in the following respects:
(1) Stabilization of pH:
This protects the higher plants and micro-organisms form direct adverse and injurious effects of sudden change in soil reaction.
(2) Amount of amendments necessary to correct the soil reaction:
The greater is the buffering capacity of soil the smaller will be the amount of the amendments required such as lime, sulphur etc. to correct the acidity or alkalinity.