In this article we will discuss about:- 1. Introduction to Buffer Systems of Blood 2. Hemoglobin Buffers 3. Chloride Shift.
Introduction to Buffer Systems of Blood:
1. Venous blood carries more CO2 than arterial blood. Hence, the pH of venous blood is more acid than that of arterial blood by 0.01-0.03 units i.e. pH 7.40 and 7.43, respectively.
2. The blood buffers consists of the plasma proteins, hemoglobin, oxy-hemoglobin, bicarbonates and inorganic phosphates.
3. When CO2 enters the venous blood, the small decrease in pH shifts the ratio of acid to salt in all the buffer pairs. When the ratio is shifted to form more of the acid, cations become available to form additional bicarbonates. In this respect, the plasma phosphates and bicarbonates play a minor role.
4. The buffering action of the plasma proteins is important because they release sufficient cations for the carriage of about 10% of the total CO2.
5. The phosphates in the red cells carry 25% of the total CO2.
6. The most important buffering role is of hemoglobin and oxy-hemoglobin which carry 60% of the CO2 of the whole blood.
Hemoglobin Buffers:
At the lungs the formation of oxy-hemoglobin from reduced hemoglobin releases hydrogen ions which react with bicarbonate to form carbonic acid. The low CO2 tension in the lung shifts the equilibrium towards the production of CO2 which is continually eliminated in the expired air:
H+ + HCO3 ↔ H2CO3 ↔ H2O + CO2
In the tissues, the oxygen tension is reduced and hence oxy-hemoglobin dissociates delivering O2 to the cells and reduced hemoglobin is formed. CO2 produced by metabolism enters the blood, where it is hydrated to form H2CO3 which ionizes to form H+ and HCO3–.
Reduced hemoglobin acting as an anion accepts the H+ ions forming acid-reduced hemoglobin (HHb). Very little change in pH occurs because the newly arrived H’ ions are buffered by formation of very weak acid.
When the blood returns to the lungs, these H+ ions are released as a result of the formation of stronger acid (oxy-hemoglobin) and the newly released H+ ion is promptly neutralized by HCO3–. This reaction is necessary for the liberation of CO2 in the lungs.
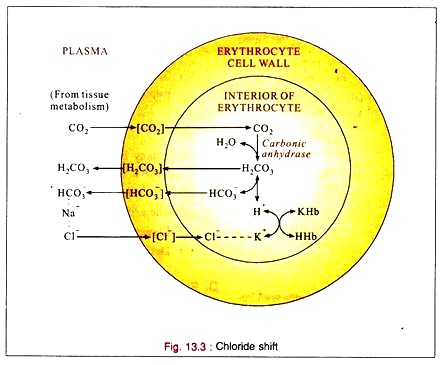
Chloride Shift:
1. CO2 reacts with water to form carbonic acid (H2CO3), mainly inside the red cell by the enzyme carbonic anhydrase present in the red cells.
2. The carbonic acid is then buffered by the intracellular buffers (Phosphate and hemoglobin) combining with potassium.
3. Bicarbonate ion also returns to the plasma and exchanges with chloride which shifts into the cell when the tension of CO2 increases in the blood.
4. When the CO2 tension is reduced, chloride leaves the cell and enters the plasma.
5. Under normal conditions the red cell is impermeable to sodium or potassium. But it is permeable to hydrogen, bicarbonate and chloride ions and intracellular sources of cation (potassium) are indirectly available to the plasma by chloride (anion) exchange. This permits the carriage of additional CO2 (as sodium bicarbonate) by plasma.
6. The CO2 entering the blood from the tissues passes into the red cells where it forms carbonic acid by carbonic anhydrase. Some of the carbonic acid returns to the plasma. The remainder reacts with hemoglobin buffers to form bicarbonate which then returns to the plasma in exchange of chloride. The chloride is neutralized by potassium in the red cells.
7. All of these reactions are reversible. At the lung, when the blood becomes arterial, chloride shifts back into the plasma, thus liberating intracellular potassium to buffer the oxy-hemoglobin and in the plasma, neutralizing the sodium which is liberated by the removal of CO2 during respiration.